Traditionally, glaucoma has been viewed as a primary optic nerve disease in which the optic nerve is damaged as a result of high intraocular pressure. This makes sense, since many patients with apparent glaucoma present in clinic with high pressure—and in most of those cases, if you lower the IOP the patient stops progressing.
Normal-tension glaucoma is also a fairly common diagnosis in a glaucoma clinic, but this particular diagnosis concerns glaucoma specialists because it’s one of the few glaucomas that doesn’t behave like the others. It tends to be more quickly progressive, difficult to treat, and can often affect central vision before peripheral fields are affected. In particular, the connection between elevated pressure and progression is sometimes missing; most patients with glaucoma will do well with an IOP of 15 mmHg or below, for example, but these patients don’t. You can try to address their problem by lowering what was already a “normal” pressure, and in many cases that will work. However, other patients will continue to show degeneration of the optic nerve.
Early in my career I decided to specialize in both glaucoma and neuro-ophthalmology, focusing on the idea that glaucoma is both an optic neuropathy and an irreversible neuro-degenerative disease. One issue that comes up when taking this approach is the need to identify normal-tension glaucoma masqueraders. These are often neurodegenerative problems such as tumors or trauma to the optic nerve that cause signs and symptoms resembling those of normal-tension glaucoma—specifically, loss of central vision in combination with visual field defects. In some cases, it’s only after standard glaucoma treatment fails to resolve the problem that it becomes apparent that glaucoma isn’t the cause of the optic nerve degeneration.
Working at our quaternary referral center’s neuro-ophthalmology clinic, I often manage patients sent to us for this exact reason; their signs and symptoms have failed to respond to standard glaucoma treatment. As a result, I’ve had a lot of experience with normal-tension glaucoma masqueraders. (I also run a very busy surgical and clinical glaucoma clinic that doesn’t specialize in neuro-ophthalmological problems, where it’s equally important to identify these patients in an efficient way.)
Here, I’d like to discuss some red flags you may encounter in your clinic that should lead you to consider the possibility that something other than normal-tension glaucoma might be causing your patient’s problem. To help illustrate these red flags, I’ll share two cases I’ve managed in the recent past.
Taking the Road Less Traveled
One concept that I find helpful when diagnosing this kind of patient is an approach to clinical reasoning laid out by a colleague at the University of Pennsylvania. This “dual-process theory” suggests that when examining a patient there are two avenues, or systems, we can follow. In System 1, the patient’s signs and symptoms are recognizable and the patient can be treated in a familiar manner. When we decide a patient fits in System 1, we proceed with our standard analysis of the details, and diagnose and treat the patient accordingly. In the case of patients with glaucoma, we ask the basic questions: Does the patient have thinning on OCT? Changes in the visual fields? Cupping? A family history of glaucoma? The answers to these questions tell us the best way to manage the patient. Managing System 1 patients allows us to see a large number of patients each day—which is currently necessary to make the practice of medicine economically feasible for any ophthalmologist.
When the patient’s presentation doesn’t fit into our standard frame-work of understanding, we switch to the alternative approach, System 2. In these cases, something we’re finding doesn’t make sense, causing us to categorize this patient as different. When we’re in System 2 mode, we’ve decided that following our standard protocol may not resolve the patient’s problem. Extra time and effort will be required to find the right treatment.
This two-system concept is a simple premise, but an important one. In-deed, it captures a fundamental aspect of the way my neuro-ophthalmology clinic runs. When I get a referral that doesn’t fit the standard glaucoma picture, it’s easy for me to convert to a System 2 line of think-ing. After all, these patients have problems that have evaded medical management. The key issue for most ophthalmologists, especially in a busy glaucoma clinic, is being alert for red flags that indicate that your patient needs to be managed as a System 2 patient.
For example, consider a 57-year-old male patient who came to me with decreased vision in both eyes. By the time I saw him, the right eye had been affected more than the left; vision in that eye had been declining for six months. After the initial onset of symptoms, he’d seen two ophthalmologists. The first was a retina specialist. The patient’s IOP wasn’t elevated, so the doctor suspected that the patient had an embolic plaque; some kind of ischemic insult had happened to the inferior portion of the retina, causing the subsequent optic nerve cupping. The patient was diagnosed with a retinal artery occlusion.
However, the patient continued to progress, which isn’t typical for a retinal artery occlusion; usually the damage is done and there’s no further progression. Given this turn of events, the retina specialist sent the patient to a glaucoma specialist, who started him on latanoprost in the right eye. Despite treatment, the patient’s vision continued to worsen in a relatively short period of time.
This is an example of an individual whose presentation and history initially placed him in System 1, until the lack of response to treatment made it clear that something other than normal-tension glaucoma must be happening. At this point he was referred to our clinic. By the time I saw the patient, I was already in System 2 mode; two expert physicians had gone through their tool boxes of differential diagnoses without resolving the problem.
 |
Examining the Patient
By the time I saw this patient, vision in his right eye was count-fingers. I usually have patients with normal tension stop their glaucoma drops temporarily to see what the baseline intraocular pressure is. (For example, if they’re off all drops and the pressure is 23 mmHg, I can make an argument that high pressure is causing the is-sues, and keep them on their drops.) In this case, the patient stopped the latanoprost and his IOPs were 18 and 17 mmHg, within the normal range.
The patient was generally in poor health; he was overweight and had hypertension, hyperlipidemia, obstructive sleep apnea and type 2 diabetes. I think it’s also fair to say that this case was complicated by the fact that this patient was not a great historian and was poorly compliant. He was from mainland India, and English wasn’t his first language, so we used a translator. He didn’t offer many details about his symptoms, simply saying, “My vision is lost in both eyes.” I asked questions, attempting to get more detail about the nature of the vision loss; he just said, “I don’t know.”
Even without more detail, serious vision loss was a red flag because most glaucoma doesn’t initially present with a decrease in vision or visual quality. Early glaucoma is usually diagnosed with a finding of cupping; patients have no idea that they’ve lost some of their peripheral vision. This patient never admitted to a blurring of vision, or poor night vision—which might lead you to suspect a cataract or something reversible. He simply said he’d had a decrease in his vision. That didn’t give me much to go on, but hearing those words from a patient immediately makes me consider optic neuropathy.
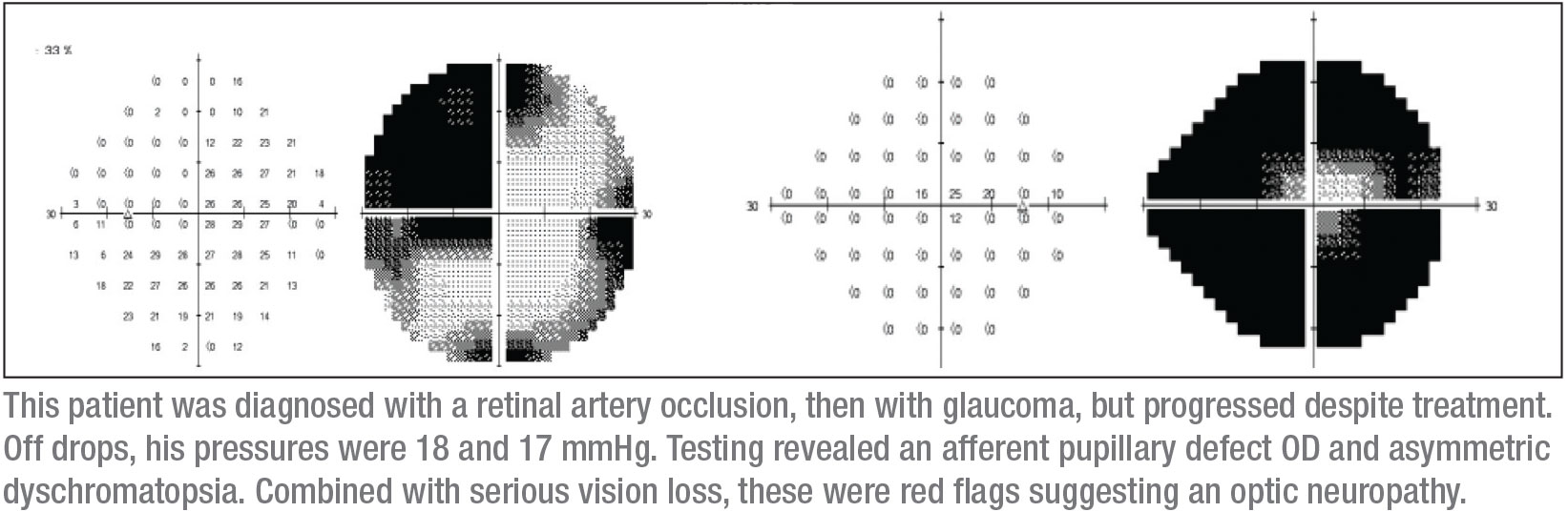
A number of things jumped out when I examined him. First, he had an afferent pupillary defect in the right eye. Second, his overall vision was poor. Third, he appeared to have a superotemporal defect in the right eye. Fourth, color testing revealed an asymmetric dyschromatopsia. (Actually, vision in his right eye was so poor that the color testing was inconclusive in that eye, but he definitely had dyschromatopsia in the left eye.)
It’s atypical for open-angle glaucoma to be asymmetric. I find asymmetry especially suspicious when it’s extreme—for example, poor vision in one eye and 20/20 vision in the other. Of course, I always perform gonioscopy when I find asymmetric disease, to look for any signs of angle recession that might cause an asymmetric glaucoma. However, I didn’t see any recession or damage to the angle in this patient.
 |
My next hallmark is visual fields, which can be critical. I started with a Humphrey visual field test, although I knew that this patient might not produce reliable automated visual field data. In fact, as expected, the visual field parameters weren’t re-liable. However, they did suggest that the right eye had a small central island of vision (as expected), and the left eye had a questionable temporal defect that didn’t respect the vertical midline. In addition, I got an OCT; it showed retinal thinning in both eyes, but more in the right eye, which was consistent with the other clinical findings.
I still felt I needed to see more-re-liable visual fields. I have the benefit of being at an academic institution where it’s fairly easy to get Goldmann visual fields—kinetic visual fields that have an operator present to explain and spend time with the patient to unmask reliable visual field defects. It was a Goldmann visual field that gave the diagnosis away; it clearly showed a superotemporal defect in the left eye, with a nearly complete visual field defect in the right eye. (See fields, p. 15.) That small circle in the middle of the right visual field is all the vision this patient had remaining in that eye.
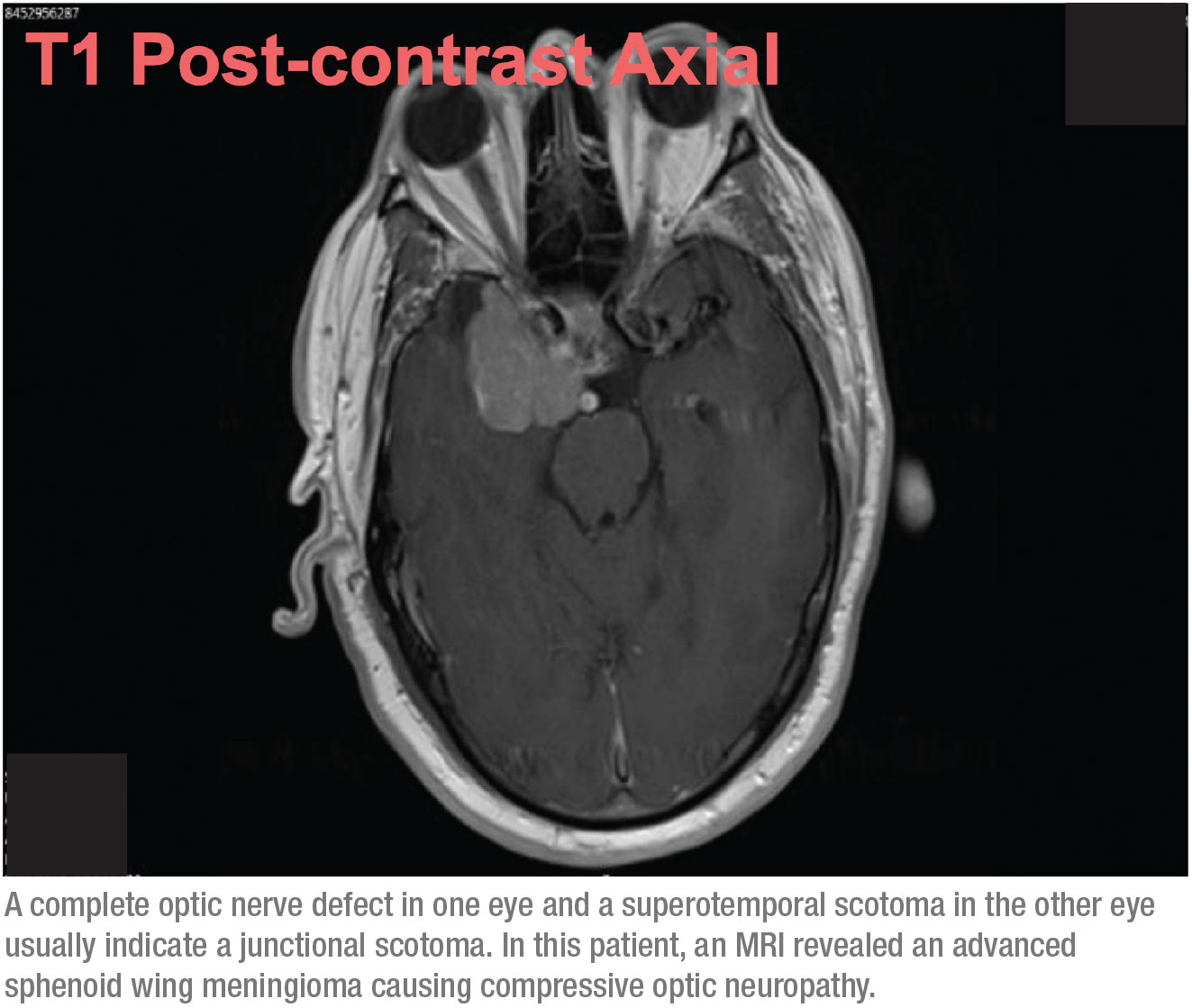
This combination of signs—a complete optic nerve defect in one eye, and a superotemporal scotoma in the other eye—usually translates to a junctional scotoma, a scotoma at the junction of the optic nerve and the chiasm, more commonly known as von Willebrand’s knee. This is an anatomical area of inferonasal nerve fibers in the optic nerve. These fibers extend the optic nerve before crossing over to the opposite optic tract. The anterior chiasmal lesion affects the optic nerve fibers and the contralateral inferonasal fibers located in von Willebrand’s knee. This will classically produce an ipsilateral optic neuropathy—in this case manifesting as a complete visual field defect and a supratemporal field defect in the contralateral eye.
When this possibility presents it-self, it’s no longer an issue of normal-tension glaucoma. This patient needed an MRI. Based on the visual fields, I knew it was very likely that we’d find a slow-growing mass in the brain such as a meningioma, causing compressive optic neuropathy. How-ever, I wasn’t expecting it to be too extensive, so I was surprised when I got a call from radiology after the MRI was done saying my patient had a midline shift, asking whether he should be sent to neurosurgery. At first I wasn’t sure we were talking about the same patient! But it turned out that the tumor was advanced. (See scan, above.)
Fortunately, since undergoing the surgery the patient has done well. Because it was a meningioma, not a malignant tumor, the surgeon was able to simply debulk it. The patient actually got a little bit of vision back in his right eye; the vision in his left eye was almost completely unaffected.
Color Vision and APDs
In addition to unusual visual fields and OCT scans, two other signs are highly suggestive of neurodegenerative problems: color vision deficits and afferent pupillary defects.
Not all retinal ganglion cells are affected equally in early glaucoma; the koniocellular retinal ganglion cells, which respond to blue/yellow stimuli preferentially, are believed to be affected first. What that means is that most color vision is typically preserved in glaucoma, even in normal-tension glaucoma patients. It’s only when the optic nerve is dead that color perception deficiency, or dyschromatopsia, is obvious from testing—at least with the Ishihara or pseudo-isochromatic color plates that we typically use in the clinic. (Less-commonly used color vision tests include the City University color vision test, the traditional D-15 dichotomous test, and the Farnsworth-Munsell 100-hue color vision test. All of these are used to look for retinal degeneration, and they test all of the colors along the visual spectrum.) Unlike glaucoma, color vision isn’t preserved with neurodegenerative disease. That makes a color vision deficit a useful tool for differentiating between optic neuropathy and glaucoma.
Two studies that have looked at the impact of glaucoma on color perception have found that the most common color deficits in patients with glaucoma are the trident, or blue/green, defects.1,2 Ironically, a limitation of the pseudo-isochromatic plates we use in clinic—including the Ishihara test—is that they don’t test for the trident defects. (In the clinic, we do have a test to stimulate these specific ganglion cells: the SITA SWAP visual field test. Unlike a standard SITA standard test, SITA SWAP uses a blue background and a yellow/orange light. That color combination can reveal defects indicative of early glaucomatous damage.) However, the color testing we typically use in clinic will pick up the deficits caused by optic neuropathies, because they affect a different set of retinal ganglion cells.
The second red flag that an optic neuropathy may be causing the optic nerve damage is an afferent pupillary defect. This is a useful thing to check because an APD is rarely found in mild or early glaucoma.3
What About MRIs?
A 40-year old female presented at our clinic with dimming vision in one eye—a symptom that should always raise suspicion. She’d gone to an optometrist for glasses three years earlier, where she received a standard visual field test; the result was abnormal. She received glasses and was diagnosed by the optometrist as having glaucoma in the right eye. Notably, her dimming vision in the left eye wasn’t helped by the glasses.
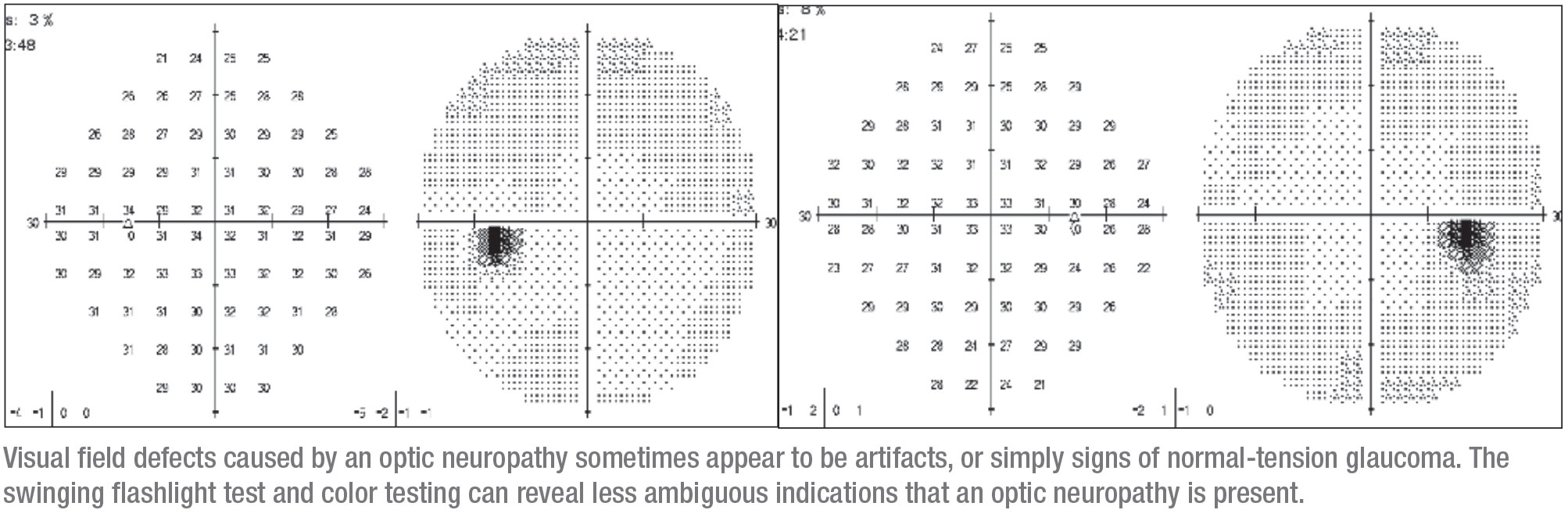
The right eye was very subtly involved. It had a tiny defect that initially could be interpreted as an artifact. This corresponded to superior thin areas on OCT, so it made sense to conclude that the problem was glaucoma and start the patient on drops. (When I saw her she’d been taking latanoprost and dorzolamide/timolol.) At the time she hadn’t been tested for a possible APD, nor was her color vision evaluated.
Before seeing me, she’d been lost to follow-up for three years. She re-turned with a more impressive visual defect in the right eye that was larger, again corresponding with thin areas on OCT. This apparent progression caused the patient to be referred to my clinic.
When I saw the patient she was basically healthy, although she had a mixed connective tissue disease. She didn’t have a family history of glaucoma. She was 20/30 OD and 20/25 OS, and after being off of the drops for a couple of weeks her IOP was 12 mmHg in both eyes. Her angles were normal and her pupils were round, but there was a sluggish APD on the right side and decreased color vision. At this point it was apparent that something other than glaucoma might be the cause of the problem, and the need for an MRI was clear.
This raises a very important question: Should a patient like this undergo an MRI of the brain or an MRI of the orbit, with special attention paid to a specific location?
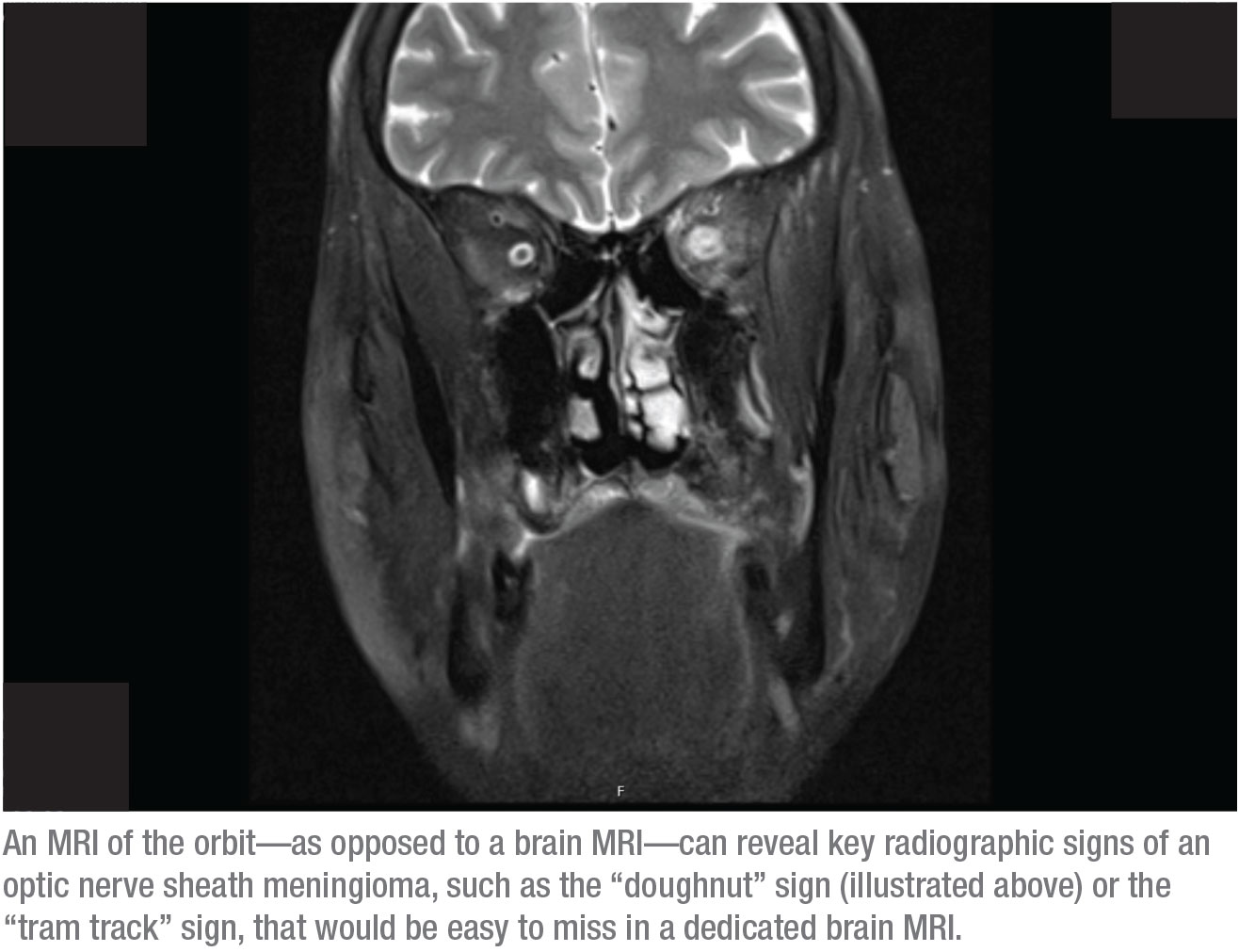
The answer is the latter—an MRI of the orbit. The reason is that key radiographic signs of an optic nerve sheath meningioma would be easy to miss on a dedicated brain MRI. In contrast, those signs can clearly be identified in an MRI of the orbit. Two of those signs, for example, are the “doughnut” sign and “tram track” sign. To see those signs, the orbit has to be fat-suppressed and the scan must show thin slices through the orbit. The only way that will be done is if you tell the radiologist that you want an orbital MRI. (I often ask for both a brain and orbital MRI, but insurance will rarely cover both.)
When an optic nerve sheath meningioma is present, the doughnut sign is usually clearly visible in the orbital MRI, because contrast is part of this scan. For example, in the scan on page 20, the white spots above the optic nerves are cross-sections of blood vessels, lit up because of the contrast in the blood. The muscles are also lit up because of the blood flow to that area, which is normal enhancement. What normally doesn’t light up is the optic nerve sheath; you shouldn’t be able to see that in an image like this. The lit-up sheath visible in this image produces the “doughnut sign” you can see in the right side of the orbit. (If we were looking at an axial cut, you might see a classic “tram track.”) The diagnosis is an optic nerve sheath meningioma.
This is the main reason you wouldn’t want to get a brain MRI of every normal-tension glaucoma patient; a brain scan might very well not reveal the correct diagnosis. (And of course, it would be a really expensive use of medical resources.)
 |
Strategies for Success
To help pick up red flags in patients who are actually suffering from a nonglaucomatous optic neuropathy in clinic, or to shift them from a System 1 into a System 2 track as early as possible, keep these strategies in mind:
• Give every normal-tension glaucoma patient a color test. Un-like visual acuity, color vision is rarely checked in a glaucoma clinic. It’s probably not necessary to check the color vision of all glaucoma patients, but patients with normal-tension glaucoma should be tested. All of my technicians are trained to check color vision, and Ishihara plates are kept in all of my exam rooms. In addition, if I become suspicious during an exam that something other than glaucoma might explain the patient’s nerve damage, I’ll do the testing myself.
• Give all glaucoma patients the swinging flashlight test before they’re dilated. Doing the test only takes two or three seconds. Of course, this should always be done for new patients, but you should also do it for returning patients. Hickam’s dictum says, “Patients can have as many diagnoses as they darn well please,” and indeed, a patient I’ve been seeing for years could unexpectedly develop a tumor, causing an APD, while also having glaucoma. A tumor is some-thing I wouldn’t want to miss.
• Be alert for steady, painless loss of vision in the history. Compressive optic neuropathy, like that caused by a tumor, is typically hard to characterize symptomatically. However, some indications in the history can be suggestive. Compressive optic neuropathy doesn’t happen quickly, unlike damage caused by ischemia, where you wake up and vision in one eye is gone. It doesn’t hurt. You just notice that vision in one eye is not as vivid, not as clear, and eventually, vision is completely lost. This may happen over a period of eight months or more.
• Poor visual acuity is a red flag. Another clue that you may not be dealing with glaucoma is that most individuals with non-glaucomatous optic neuropathy have poor visual acuity. In contrast, most types of glaucoma don’t undercut good central acuity until the very end stages. (Of course, that’s not true in every case of normal-tension glaucoma; in some patients NTG does cause central and paracentral defects that can get very close to the central 10 degrees of vi-sion and affect visual acuity and split fixation. But poor central vision is a hallmark of most non-glaucomatous optic neuropathies.)
A study done in 1982 looked at this question.4 The authors evaluated visual acuity and optic nerve injury after different kinds of insults to the nerve, including glaucoma, ischemia (from something like nonarteritic ischemic optic neuropathy), compression, and toxic optic neuropathy from drugs like amiodarone or ethambutol. They looked at the end-stage visual acuity and correlated that with the vertical cup size and nerve area, as well as the number of axons remaining. What they found was that most glaucoma patients had visual acuity between 20/25 and 20/400, depending on the stage of the disease. On average, these patients had large optic nerve heads and had more retinal ganglion cells than patients who had undergone things like ischemia or compressive or toxic optic neuropathy.
• Check the color of the nerve rim. Nicholas J. Volpe, MD, a co-author of the neuro-ophthalmology textbook that I learned from as a resident and fellow, and still refer to today, often described pallor ex-tending beyond the cup into the rim of the nerve. When any part of the optic nerve rim is pale, that’s a sign that glaucoma may not be the only problem, because in most glaucomas, no matter how advanced, the optic nerve rim remains pink. It might be extremely thin because of cupping, but it usually remains a healthy color until the end stages of the disease. If you see a rim that almost looks the same color as the cup itself, that’s a warning sign to enlarge your list of possible causes.
• If a patient is coming in just for a pressure check or visual field, make sure they’re also checked for an APD. Since these patients aren’t being dilated, it’s an excellent opportunity to make sure nothing neurological has changed.
• At the least, make sure new glaucoma patients are checked for an APD and color vision deficiency. When a new glaucoma patient comes in, before doing my gonioscopic exam, I look at the color tests that have been done by my technician and check for an APD.
• If you encounter a red flag, don’t hesitate to get a second opinion. If there’s any question in your mind as to whether your patient might have a problem besides glaucoma, asking a colleague can be a valuable option. I frequently hear from col-leagues asking my opinion about whether a patient’s fields suggest a neurological issue. Getting a second opinion is always a good idea.
Stay On the Lookout
My experience has shown that a good number of patients diagnosed with normal-tension glaucoma actually have a different problem. How-ever, I think it’s important to point out that this doesn’t mean that the doctor making the normal-tension glaucoma diagnosis did something wrong. It could be simply that some key findings in the physical exam—findings that might have shifted the patient into a System 2 track—were either overlooked, or the relevant data wasn’t collected. In this situation, the need for a System 2 approach may only become apparent when the patient fails to respond to standard glaucoma treatment.
 |
Because I also run a busy glaucoma clinic, I totally understand when my colleagues tell me they’re seeing 50 to 80 patients a day and there’s no way they can do color testing on every patient. And even with the best of intentions, some patients end up dilated before the ophthalmologist sees them, so you can’t always check for an APD. Nevertheless, these things are worth trying to fit into your patient flow, especially when you’re dealing with a patient with normal-tension glaucoma. Even without testing for color deficiencies and APDs, looking carefully at the visual fields with the left and right eyes side-by-side will often reveal something suggesting a neurological pathology.
I’m not suggesting that every ophthalmologist treating glaucoma should be able to diagnose a junctional scotoma or an optic nerve sheath meningioma. The important thing is to recognize a red flag and realize that this patient is different from the others. REVIEW
Dr. Ross is an assistant professor, dual-appointed in the departments of neurology and ophthalmology at the Scheie Eye Institute and Hospital of the University of Pennsylvania. She has had fellowship training in glaucoma and neuro-ophthalmology.
1. Pacheco-Cutillas M, Edgar DF, Sahraie A. Acquired colour vision defects in glaucoma—their detection and clinical significance. Br J Ophthalmol 1999;83:12:1396-402.
2. Sample PA, Boynton RM, Weinreb RN. Isolating the color vision loss in primary open-angle glaucoma. Am J Ophthalmol 1988;15:106:6:686-91.
3. Charalel RA, Lin HS, Singh K. Glaucoma screening using relative afferent pupillary defect. J Glaucoma 2014;23:3:169-73.
4. Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol 1982;
100:135–146.



