Glaucoma and elevated IOP are common but challenging complications to wrangle following corneal transplantation. They may arise from various factors such as synechiae, inflammation and steroid use, impacting keratoplasty outcomes. Here, I’ll explore strategies to control high pressures and minimize damage to the optic nerve, and discuss the importance of coordinated care between cornea and glaucoma specialists.
Risk Factors and Mechanisms
The incidence of glaucoma after penetrating keratoplasty is reported to range from 9 to 31 percent in the early postoperative period and from 18 to 35 percent in the late postoperative period, with incidence variation owing to differing definitions of glaucoma.1 Causes of high IOP and glaucoma after PK include angle distortion and collapse of the trabecular meshwork due to tight, long, superficial sutures; large-diameter trephine use; same-sized or undersized grafts; retained viscoelastic; inflammation; steroid response; PAS formation; and exacerbation of pre-existing glaucoma. The last two of these are among the leading causes of increased intraocular pressure or glaucoma following a PKP procedure.
Steroid response and pre-existing glaucoma are the leading causes of high IOP and glaucoma after endothelial keratoplasty. Other causes include higher preoperative IOP, air bubble-induced angle closure, retained viscoelastic, PAS formation and inflammation. Additionally, certain EK indications, such as bullous keratopathy, are also associated with an increased risk of glaucoma and glaucomatous progression than other conditions.2 IOP elevation after lamellar keratoplasty has been reported to occur at a lower incidence than following PK, potentially a result of less surgically induced angle and trabecular meshwork damage and a reduced need for postoperative steroids.3 A study of 1,657 eyes reported the 10-year probability of glaucoma-related vision loss to be 1 percent after EK, 2.1 percent after ALK and 3.6 percent after PK (p=0.036).3
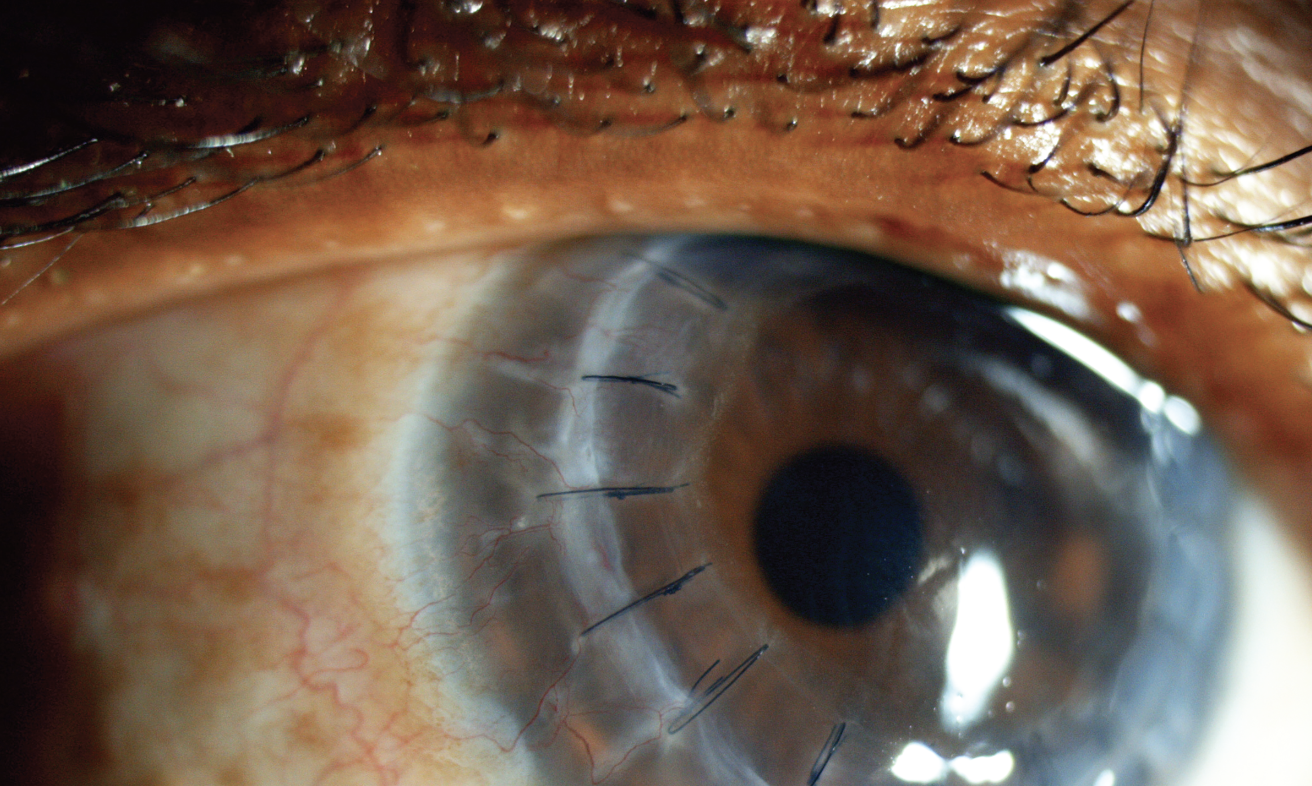 |
| For penetrating keratoplasty, using a slightly oversized full-thickness graft and placing sutures evenly may have some IOP-related benefits. For EK procedures, be sure to place an iridotomy inferiorly. Photo: Science Source Images. |
Avoiding Complications
Corneal surgeons must pay extra attention to those patients who go into surgery with established glaucoma. These are the patients who are most likely to have problems with pressure and progressive glaucoma after surgery. Gonioscopy is a must preoperatively to establish if the risk factor of PAS is present, and it should be performed periodically after the corneal transplantation, especially if IOP rises.
When performing PK, using oversized donor tissue may confer some IOP-related benefits. Additionally, ensure sutures are evenly placed and avoid compressing the angle. In EK procedures, acute angle closure can occur because of the air or gas bubble, so it’s important to make an iridotomy. Place it inferiorly to avoid occlusion by the bubble when the patient is upright.
Medical Management
Management of keratoplasty-induced glaucoma or high IOP requires careful monitoring in the immediate postoperative period. Perform frequent pressure checks to catch acute IOP increases and treat with topical medications. For chronic pressure increases due to steroids, taper off the steroids as quickly as is safely possible while balancing the risk of a graft rejection. Initiate topical drops as necessary. If the view of the angle is good, laser trabeculoplasty can be performed.
Managing topical glaucoma therapy requires careful consideration to both the glaucoma and corneal health. High pressures need to be managed, but many topical glaucoma medications have some degree of corneal toxicity. This toxicity may irritate the cornea while a PK graft re-epithelializes in the immediate postoperative period. Pilocarpine 4% and brimonidine 0.1% and 0.15% were reported to induce 60-percent epithelial cell death at four hours; pilocarpine 2% and latanoprost 0.005% resulted in nearly 100-percent toxicity after 16 hours; and timolol 0.5% and pilocarpine 1% induced 40-percent cell death at 24 hours.4
Switching to or initiating preservative-free topical medications can help avoid punctate epitheliopathy or other ocular surface irritation. Some BAK-preserved alternatives include preservative-free timolol, brimonidine preserved with Purite and travoprost preserved with Sofzia. Lamellar keratoplasty usually leaves the epithelium intact and glaucoma drops can be continued.
Reports of carbonic anhydrase inhibitors contributing to graft or corneal failure are rare, but if a graft has been in place for a long time and has significant endothelial cell loss, maintain suspicion of the CAI if the graft develops thickening or edema in the absence of rejection. Stop the CAI and observe whether the edema reverses. A recent patient of mine who didn’t have a corneal transplant experienced vision loss and her cornea was noted to be very thick with mild Descemet’s folds. After stopping the CAI and substituting with another medication, her cornea returned to normal, and her vision improved. She was happy that the solution involved switching medications rather than a step up in therapy.
Surgical Management
If the patient’s pressure can’t be controlled medically, surgery should be done to protect the optic nerve. Nerve damage can occur rapidly if pressures are high, so it’s important to examine the nerve periodically.
Tube shunts are commonly performed in PK patients, often because the conjunctiva is scarred already in these eyes, perhaps due to past trauma or past surgery. If the conjunctiva is healthy, trabeculectomy is a suitable approach, especially given tube shunts’ association with higher rates of endothelial loss, which puts the graft at risk for failure.
When implanting a tube shunt in a patient who’s had a corneal transplant, ensure the tube enters below Schwalbe’s line. Tube entry anterior to Schwalbe’s line is a risk factor for endothelial cell loss. If the patient is pseudophakic, sulcus placement is recommended to keep the tube far from the corneal endothelium.
Co-managed Care
It’s important to stay in contact with the patient’s co-managing specialist. Glaucoma specialists should be aware of and be prepared to check for signs of corneal graft rejection and communicate these occurrences with the cornea specialist.
Also be sure to counsel patients about the need for two specialists. Many patients may be confused why they have to see two different doctors. They may have questions about the number of visits needed or it may be burdensome to attend so many appointments.
Discuss care with the co-managing specialist. Will they completely cede the follow-up of the optic nerve and visual fields to the glaucoma specialist, or will the cornea specialist also check for those things? When there are two clinicians managing a patient who doesn’t want to have to come for all those visits, it’s easy to assume the other clinician will do the dilating, for example. There needs to be a clear understanding of who’s going to do what for the patient.
Dr. Giaconi is a glaucoma specialist in the Glaucoma Division at the UCLA Stein Eye Institute. She has no related financial disclosures.
1. Mihail Z and Alina-Cristina A. Glaucoma after penetrating keratoplasty. Romanian J Ophthalmol 2017;61:3:159-165.
2. Saini C, Davies EC, Chodosh J, Shen LQ. Glaucoma in patients with endothelial keratoplasty. Cornea 2022;41:12:1584-1599.
3. Borderie VM, Loriaut P, Bouheraoua N, et al. Incidence of instraocular pressure elevation and glaucoma after lamellar versus full-thickness penetrating keratoplasty. Ophthalmol 2016;123;7:1428-1434.
4. Robciuc A, Ruokonen S, Witos J, et al. Toxicity of glaucoma drugs on corneal epithelial cells. IVOS 2016;57:4384.



