|
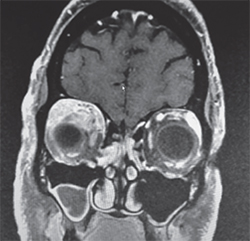
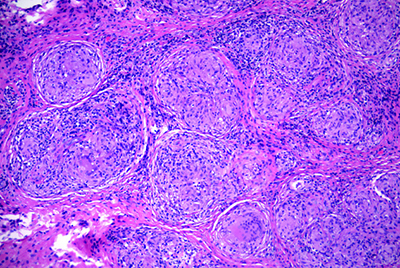
On follow-up, the patient’s exam remained stable. His lab workup was negative, although it consisted of tests with generally poor sensitivity for orbitally limited diseases. Therefore, given his unrevealing laboratory studies and concerning clinical exam and imaging, the differential diagnosis remained broad and included poorly circumscribed lesions, such as idiopathic inflammation; specific inflammatory conditions (sarcoid, Wegener’s, Sjögren’s, etc.); metastatic lesions; lymphoproliferative disease; and vascular lesions. The concern for a neoplastic etiology and unclear diagnosis prompted orbitotomy with lesion biopsy and mass debulking though a lid crease approach. Fresh tissue was sent for flow cytometry, which showed no evidence of lymphoproliferative disease. However, the histopathology demonstrated non-caseating granulomatous inflammation consistent with sarcoidosis (See Figure 3).
Prior to the initiation of prednisone, the patient underwent a computerized tomography scan of the chest that demonstrated hilar lymphadenopathy and interstitial inflammation, concerning for pulmonary sarcoid. Quantiferon Gold antibody testing ruled out tuberculosis. Referral to pulmonology demonstrated normal pulmonary function testing and dermatology referral led to a biopsy of the patient’s facial rash. This revealed psoriasiform dermatitis without granulomatous inflammation. The patient was also referred to rheumatology for definitive therapy.
While rheumatology input was pending, the patient was diagnosed with sarcoidosis and started on 60mg daily of oral prednisone. He had significant improvement following surgical debulking and corticosteroids. Two weeks postop, his proptosis, hypoglobus and extra ocular motility had improved (See Figure 4). There was persistent right upper lid ptosis. Over two months time, he tolerated an oral prednisone taper to 5 mg daily, and is awaiting evaluation by his rheumatologist to determine the need for long-term immunosuppression.
Discussion
Sarcoidosis is a multi-system disease of non-caseating granulomatous inflammation thought to be the result of immune stimulation by self or non-self antigens.1 Despite investigation of potential etiologies, including a multitude of genetic, infectious and environmental factors, there have been no established causative relationships. Regardless of the inciting antigen, the final common pathway of disease is thought to result from an exaggerated immune response resulting in a T helper cell 1 immune cascade with the subsequent elaboration of chemokines and cytokines, resulting in organ fibrosis and dysfunction.1
|
Pulmonary involvement is most common among patients with sarcoidosis, occurring in more than 90 percent of patients with sarcoidosis.1 However, this disease also frequently manifests in the lymph nodes, skin and eyes.4 A comprehensive review of the clinical features of sarcoidosis discusses the dermatologic disease manifestations as two discrete classifications: nonspecific lesions that are inflammatory skin reactions, most commonly erythema nodosum; and specific lesions that demonstrate granulomatous inflammation on biopsy. Specific lesions are commonly firm, 2- to 5-mm papules that are translucent red-brown or yellow-brown in color; however appearance is very variable4 and may include plaques, psoriaform lesions and intradermal nodules, among others. Lupus pernio is a disfiguring form of facial sarcoidosis that may be severe enough to erode into bone. Interestingly, skin lesions have a predilection for involvement of scars, tattoos, skin piercings and sites of old trauma.4 In this case, psoriaform dermatitis was present, but there was no evidence of granulomatous inflammation to suggest this rash was a specific lesion of sarcoidosis.
The most common ophthalmologic manifestation of sarcoid is uveitis, present in 70 percent of patients with ocular involvement.1 Orbital involvement is much rarer; in a review of 379 cases of ocular sarcoidosis at Henry Ford Hospital, only 30 cases demonstrated orbital and/or adnexal involvement. Of these, only nine cases involved the orbit, eyelids and extraocular muscles.4 The majority of orbital lesions were situated in an anterior, superior position, as seen in this case.
Interestingly, in this case the patient had disease manifestations in all three of the aforementioned organ systems at the time of diagnosis. It is unclear from the current literature how frequently patients present with pulmonary, dermatologic and ocular findings simultaneously. Given the reported frequency of each organ system’s involvement, the incidence of all three occurring likely ranges from 1 to 23 percent of patients.4 Nonetheless, a large case-control study investigating the clinical characteristics of patients with newly diagnosed sarcoidosis illustrated that it is relatively rare for patients to present with disease in three organ systems. Among the 736 studied patients, only 13 percent had disease involving three organ systems at the time of diagnosis.5
On the other hand, 50 percent of patients will have single-organ involvement at presentation.5 In fact, ocular sarcoid may pose a diagnostic dilemma, as it is not uncommon for the systemic and laboratory workup to be unrevealing. Short of biopsy, no clear diagnostic criteria for definitive diagnosis have been established. In cases of systemic involvement, chest radiography demonstrating hilar adenopathy and an elevated ACE level are suggestive of sarcoidosis. However, CT scan of the chest has been found to have increased diagnostic sensitivity.4
The mainstay of therapy for sarcoidosis remains immunosuppression with corticosteroids.1 However, in the aforementioned review of patients with orbital sarcoid, surgical debulking is also used to supplement systemic therapy with good long-term outcomes.6 At present, there are no randomized controlled trials comparing therapeutic treatment options in systemic sarcoidosis; however, steroid-sparing immunosuppressants are widely used.
| The most common ophthalmologic manifestation of sarcoid is uveitis, present in 70 percent of patients with ocular involvement. |
In conclusion, ocular sarcoidosis remains a great masquerader in ophthalmologic disease given its diverse manifestations, and should be considered in cases when inflammatory and infiltrative diagnoses are considered. Likewise, clinical suspicion should remain high despite initial unremarkable ACE level and chest radiography as, in this case, these tests are often negative in ophthalmologic disease. Additionally, the threshold for checking a CT scan of the chest should be low, as it is a more sensitive test than an X-ray. Sarcoidosis responds well to systemic immunosuppressive therapy, but care must be taken to rule out all possible neoplastic and infectious etiologies prior to trial of systemic immunosuppression. Further, consultation of a rheumatologist should be sought to entertain initiation of steroid-sparing agents and for evaluation and treatment of systemic disease. REVIEW
The author would like to give special thanks and acknowledgement to Brian Doyle, MD, Ralph C. Eagle, MD, and Michael P. Rabinowitz, MD.
1. Umur KA, Tayfun B, Oguzhan O. Different ophthalmologic manifestations of sarcoidosis. Curr Opin Ophthalmol 2012;23:447-484.
2. Rothova A. Ocular involvement in sarcoidosis. BrJ Ophthalmol 2000;84:110-116.
3. Rybicki BA, Iannuzzi MC. Epidemiology of sarcoidosis: Recent advances and future prospects. Semin Respir Crit Care Med 2007;28(1):22-35.
4. Demirci H, Christianson MD. Orbital and adnexal Involvement in sarcoidosis: Anaylsis of clinical features and systemic disease in 30 cases. Am J Ophthalmol 2011;151(6):1074-1080.
5. Baughman, RP, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001;164(10): 1885-1889.
6. Judson M. The clinical features of sarcoidosis: A comprehensive review [published online ahead of print October 02 2014]. Clin Rev Allerg Immunol 2014 doi 10.1007/s12016-014-8450-y.