Prior to antibiotics, surgery was not elective, but a last resort for patients who suffered from various illnesses, as it usually led to lethal infections. While antibiotics effectively eliminate or reduce postoperative infections, certain infections, such as those caused by methicillin-resistant Staphylococcus aureus, still prove difficult to treat with conventional antibiotics, as the bacteria have evolved strong resistance mechanisms.
Refractive surgery and cataract surgery are becoming more common worldwide; at the same time, a growing number of people suffer postoperatively from MRSA. In the 1950s, only 1 to 2 percent of all Staph infections were MRSA, whereas today, that number has risen to 50 percent.1 As its name suggests, this infection is resistant to methicillin and other beta-lactamase antibiotics, but it's also increasingly resistant to fluoroquinolones, the most commonly prescribed class of perioperative antibiotics for ocular prophylaxis. The virulence of MRSA is exacerbated by its ability to inhibit our innate immune response by recruiting neutrophils to the infection site and lysing them. For these reasons, the visual outcome of many patients is significantly worse than 20/20. Minimizing the risk of patients contracting intraoperative infections, as well as maximizing the success of treatment, is therefore of utmost concern. In this article, we'll take a look at the extent of the MRSA problem and what can be done to counter antibiotic resistance.
Visual Implications of MRSA
The most important outcome of an ocular infection is a patient's final visual function. Generally, visual outcome following MRSA is particularly poor. MRSA infection often results in tissue necrosis and sloughing of the epithelium and stroma, all of which lead to poor vision. Once host defenses begin to halt tissue destruction and phagocytize the debris, epithelial resurfacing begins. Fibroblasts, derived from histiocytes and keratocytes, form scar tissue, which results in ocular opacity and scarring.2
Clinical studies of MRSA patients have been conducted to determine the possible visual outcomes. One such study focused on patients who presented to a
Another retrospective study looked at the visual outcomes of 12 patients infected with MRSA following LASIK or PRK across 10 cornea and refractive practices between May 2002 and February 2005. Postoperatively, 10 of the 12 patients (13 eyes) were given fluoroquinolones, and the other two were given tobramycin and erythromycin. BCVA at the time of publication of the manuscript ranged from 20/25 to 20/150, averaging 20/60. Unfortunately, two cases required excision of the necrotic flap and two patients underwent penetrating keratoplasty to treat the infection. At publication time, four more eyes were pending a PKP procedure and one was pending phototherapeutic keratectomy. In total, six of the 13 eyes (46 percent) required keratoplasty following treatment to better regain visual function.4
In a separate study which reviewed all published literature from 1991 to 2003, 103 cases of post-LASIK infectious keratitis (87 patients) were identified. Ultimately, only 15 of the 103 eyes (14.5 percent), required keratoplasty. This study doesn't report which of the 103 infected eyes were methicillin-resistant; however, there is a clear discrepancy between this rate and the former investigation. Final visual acuity was also measured. Gram positive infections averaged a final Snellen VA of 20/45, fungal infections averaged 20/297 and mycobacterium averaged 20/55.5 It's clear that due to the high virulence, MRSA is difficult to effectively treat unless it's discovered during an early phase of infection and the antibiotics initially used happen to be effective against the particular strain.3 But what is it about MRSA that makes it so virulent?
Becoming Virulent
The number of MRSA present in relation to the population levels of other bacterial species may play a role in instigating virulence. The concept of quorum-sensing describes the regulation of gene expression in response to cell density, allowing the bacteria to adapt to changing environmental conditions.6 Bacteria sense the surrounding cell density via the secretion of molecules known as auto-inducers. As the bacterial population grows, so does the level of auto-inducers. At some threshold, the auto-inducers trigger gene activation, resulting in a bacterial signaling cascade, and the production of virulence factors ensues.7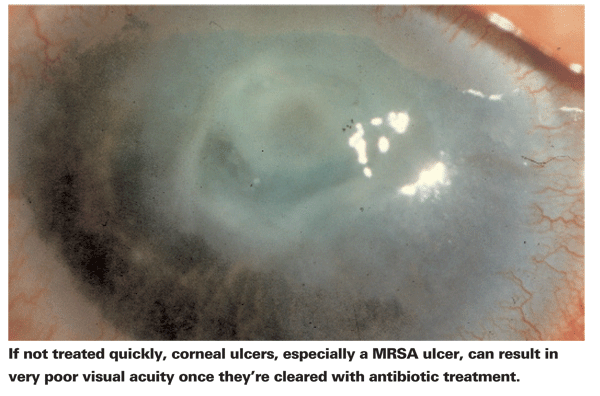
In S. aureus, one of these virulence factors is a complex pro-inflammatory peptide called phenol-soluble modulin (PSM-peptide). The production of PSM peptides is strictly controlled by the bacteria's accessory gene regulator (agr) system, which regulates most of the known aggressive virulence factors.6 The PSM-peptides secreted by S. aureus lure neutrophils to their proximity, activate them and then lyse them—effectively eliminating the body's cellular defense against the bacteria.8 Because of its strong alpha-helix structure, PSM-alpha-3 can infiltrate and break the neutrophils' cell membranes.
Intensifying this neutrophil lysing problem is the tendency of fluoroquinolones to accumulate inside the neutrophils, which carry the antibiotics to the infection site. Emerging multi-resistant strains of MRSA proficiently inhibit neutrophil chemotaxis to the infection site.9,10 Any fluoroquinolones that arrive at the infection site are simply inhibited via mutations in the antibacterial target site.11,12 With minimized quantities of antibiotics present, the bacteria can flourish, and the immune infiltration results in subsequent scarring and poor visual outcomes.
Evolution of Resistance
The human body is a symbiotic home to millions to bacteria cells, most of which never cause infection. Instead, they aid in such useful jobs as food digestion and immune system function. Surprisingly, the MRSA bacterium is found in the normal flora of a quarter of the population. These people usually live complication-free until an opportunity, such as an abrasion and break in the corneal epithelium, allows for an infection. The remaining non-MRSA carriers can become infected, for example, when an open wound comes in contact with MRSA. However, the carriers are at much higher risk for infection.13 There is currently no explanation as to why some people carry MRSA and others do not.
When infection occurs, we turn to antibiotics. Bacteria have not ignored this challenge for survival, and they've evolved several mechanisms that provide them with antibiotic resistance. There are four known mechanisms of resistance: enzymatic inactivation of the antibiotic, which is the most common method used by bacteria; physical removal of the antibiotic from the cell via an efflux pump; modification of the antibiotic target site and production of an alternate metabolic pathway that bypasses the action of the drug.14
One common misconception in treating bacterial infections surrounds fourth-generation fluoroquinolones. These fluoroquinolones inhibit both the bacteria's DNA gyrase and topoisomerase IV, both of which normally aid in bacterial replication by unraveling DNA supercoils. It was believed that in order for bacteria to adapt to fluoroquinolones, two separate genetic mutations of the bacteria were required. As this is statistically unlikely, topical fourth generation fluoroquinolones were believed to be the most effective method of treatment.
Unfortunately, fluoroquinolone resistance can also be generated via the development of efflux pumps, which are highly evolved transport proteins designed to actively pump multiple antibiotics and anti-bacterial agents out of the bacteria.15 Fortunately, antibiotics that effectively shut down these pumps are in the development phase, and could prove very useful. These new drugs may provide better treatment options, leaving more patients with better visual outcomes following the treatment.
Measuring Resistance
The incidence of MRSA seems to parallel that of other infectious diseases and immune deficiencies, and is particularly common in hospitals.16 A S. aureus infection that occurs within a hospital is approximately four times more likely to be resistant to antibiotics than an infection that occurs outside of medical facilities.13 The large population of unhealthy patients in close contact with each other, surgery with the presence of open wounds and the lack of proper hygiene all allow infection to breed. With the increase in the number of cataract and refractive procedures being performed, a corresponding rise in hospital-acquired MRSA is to be expected.17
One of the most important methods for measuring antibacterial effectiveness is the Minimum Inhibitory Concentration value. The MICx is defined as the minimum concentration that inhibits the growth of x percentage of the tested isolates. MICs have proven to be valuable measures of resistance, but their usefulness is directly linked to the methodology used to obtain them. Although not a perfect system, the accepted method of testing MIC is by using saline water systems in Petri dishes. No matter how much scientists try, it's impractical to expect perfect results from in vitro testing compared to in vivo applications.18
MRSA likely developed from the penicillin-resistant strains of S. aureus, which were first encountered in 1944.19 It's important to note that methicillin-resistant and methicillin-susceptible (MSSA) S. aureus are quite different. The Ocular Tracking Resistance in
Clinically, certain people (about a third of MRSA patients) have multi-resistant strains of MRSA, but their incidence appears to be random. Unfortunately, these multi-resistant patients usually display worse visual outcomes than regular MRSA patients due to the difficulty of treatment.20
In contrast, trimethoprim has been found to be 93.9 percent effective against MRSA isolates.21 Trimethoprim is a 20-year-old antibiotic that is now rarely used, but was once commonly prescribed as Polytrim, a combination of polymyxin B and trimethoprim. The high susceptibility with trimethoprim suggests a possible future treatment paradigm for cycling between different anti-infective agents.
Unlike MRSA, MSSA is easily treated with similar antibiotics. The fluoroquinolones carry an 80-percent susceptibility rate for MSSA infections. Furthermore, MSSA infections are 98 percent susceptible to trimethoprim.21
Fighting MRSA
The overuse of antibiotics has allowed MRSA to quickly and efficiently become immune to mainstream antibiotics such as penicillin. As noted above, one method of slowing resistance is cycling between different classes of antibiotics. Cycling may also accelerate recovery and increase final visual function. When cycling between two or more different antibiotic agents, the antibiotics should be from different classes, e.g., from aminoglycosides and fluoro-quinolones. Depending on the pattern of resistance that a certain kind of bacteria displays, cycling can be applied to prophylactic antibiotics with several months between cycles.22
Another method of controlling resistance works by actually modifying bacterial resistance itself. To become resistant to methicillin, S. aureus altered its penicillin-binding protein, the target site for beta-lactam antibiotics.23 New beta-lactam antibiotics, namely carbapenems, have potent anti-MRSA activity because carbapenems have excellent affinities to the PBP of MRSA, even ones that have been altered to become resistant to the fluoroquinolones. These carbapenems bind to the PBP of the bacteria, lysing the otherwise resistant bacteria. While these drugs are awaiting clinical approval, they appear to be quite powerful in laboratory testing, showing low MIC against even highly resistant strains of MRSA.5
All told, MRSA is a dangerous infection, and effective treatment depends on rapid, efficient recognition. With new advances such as the discovery of agr and quorum-sensing as the possible catalysts of pathogenesis, further research looks promising. Researchers must stay ahead of the curve with regards to bacterial evolution, as the potential for postoperative ocular infections will always be a problem. It's doubtful that a "perfect" treatment will be developed; however, scientists and physicians must keep developing new and more powerful therapies and treatment protocols that fight off even the most serious and most evolved MRSA infections.
Dr. Abelson, an associate clinical professor of ophthalmology at
1. Sohnle PG. On-line article: MRSA infections on the rise, but treatable if caught early.
2. Ang LPK, Azar DT. Ocular surface epithelial stem cells and corneal wound healing response to injury and infection. In: Albert DM, Miller JW, eds. Albert and Jakobiec's Principals and Practice of Ophthalmology, 3rd ed. Canada: Saunders Elsevier, 2008:481-482.
3. Deramo V, Lai JC, Winokur J, et al. Visual outcome and bacterial sensitivity after methicillin-resistant Staphylococcus aureus-associated acute endophthalmitis. Am J Ophthalmol 2008;145:3:413-417.
4. Solomon R, Donnenfeld ED, Perry HD. Methicillin-resistant Staphylococcus aureus infectious keratitis following refractive surgery. Am J Ophthalmol 2007:143:4:629-34.
5. Chang MA, Jain S, Azar DT. Infections Following Laser in situ Keratomileusis: An Integration of the Published Literature. Surv of Ophthalmol 2004;49:269-280
6. Otto M. Quorum-sensing control in staphylococci – a target for antimicrobial drug therapy. FEMS Microbiol Lett 2004;241:2:135-41.
7. Schauder S, Bassler Bl. The lauguage of bacteria. Genes Dev 2001;15:12:1468-1480.
8. Wang R, Braughton KR, Kretschmer D. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nature Medicine 2007;13:12:1510-14.
9. Foster T. Immune evasion by staphylococci. Nature 2005;3:48-58.
10. Iihara H, Suzuki T, Kawamura Y, et al. Emerging multiple mutations and high-level fluoroquinolones resistance in methicillin-resistant Staphylococcus aureus isolated from ocular infections. Diagn Microbiol Infect Dis 2006;56:297–303.
11. Walters JD, Zhang F, Nakkula F. Mechanisms of fluoroquinolone transport by human neutrophils. Antimicrobial Agents and Chemotherapy 1999:2710–2715.
12. Palazzolo-Balance A, Reniere M, Braughton K, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. The Journal of Immunology 2008;180:500–509.
13. Kanajani ZA, Fowler VG. Infecciones por Staphylococcus aureus: Nuevos retros para un viejo patogeno. Enfermedades Infecciosas Microbiogia Clinica 2006;24:182-93.
14. Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med 2006;119:6:S3-10; discussion S62-70.
15. Webber MA, Piddock LJV. The importance of efflux pumps in bacterial antibiotic resistance. J Antimicrob Chemother 2003;51:9-11.
16. Chambers HF. The changing epidemiology of Staphylococcus aureus? Center for Disease Control and Prevention's Emerging Infectious Diseases, 2001;7:2.
17. Zetola N, Francis JS, Nuremberger EL, et al. Community-acquired methicillin-resistant Staphylococcus aureus: An emerging threat. Lancet Infect Dis 2005;5:275-286.
18. Andrews J. Determination of minimum inhibitory concentrations. J Antimicrob Chemother 2001;48:S1:5-16.
19. Kirby WMM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science 1944;99:452-453.
20. Mino H, Koss MJ, Blumenkranz MS, et al. Antibiotic susceptibility of preoperative normal conjunctival bacteria. Amer J Ophthalmol 2005;139:4.
21. Asbell PA, Colby KA, Deng S, et al. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates.
22. Schentag JJ, Hyatt JM, Carr JR, et al. Genesis of methicillin-resistant Staphylococcus aureus (MRSA), how treatment of MRSA infections has selected for vancomycin-resistant Enterococcus faecium, and the importance of antibiotic management and infection control. Clin Infect Dis 1998;26:1204-1214.
23. Spratt BG. Resistance to antibiotics mediated by target alterations. Science 1994;264:388-93.



