Neovascular glaucoma—a progressive, secondary glaucoma with numerous causes—can be a challenging disease to treat, given that it involves underlying causes not present in many other forms of glaucoma. It may begin with neovascularization of the iris or angle in the presence of normal intraocular pressure; in the late stages of the disease, we find a closed anterior chamber angle and markedly elevated intraocular pressure. The disease is also characterized by increasing inflammation of the anterior segment as it progresses.
Neovascular glaucoma has been associated with more than 40 other diseases, including diabetes mellitus, central retinal vein occlusion and ocular ischemic syndrome.1 In particular, about 97 percent of neovascular glaucoma cases appear to be associated with retinal ischemia. Retinal ischemia triggers the release and diffusion of vascular endothelial growth factor, interleukin and other angiogenic factors. These factors can cause neovascularization in the iris and anterior chamber angle, which then obstructs the trabecular meshwork and causes peripheral anterior synechiae formation, reducing aqueous outflow and producing elevated IOP. For this reason, treating the underlying ischemia is a key part of managing the disease.
However, eliminating the cause of the angiogenesis may not lower the IOP, especially if the angle is closed. Use of an anti-VEGF drug such as bevacizumab may aid regression and thereby reduce IOP; other options include aqueous suppressants, osmotic drugs (except for glycerol in diabetic individuals), topical cycloplegics and corticosteroids. However, surgical options are often the method of choice for lowering IOP—especially when there is synechial closure of the anterior chamber angle.
Surgical Options
Surgical treatment for neovascular glaucoma has evolved from cyclodestructive procedures to full-thickness filtration surgery, to trabeculectomy with an-tifibrosis drugs and glaucoma drainage implant surgery. The choice of a surgical approach may be influenced by several factors, including the stage of the disease. Many surgeons favor drainage implants when the disease is more advanced or when severe inflammation is present, which would be associated with a poorer prognosis if trabeculectomy were chosen. Other factors that influence the choice of surgery include underlying etiology (surgical therapy is not indicated in the presence of a malignant ocular tumor, for example); visual potential (less-invasive procedures would be favored in eyes with limited visual potential); and severe comorbid conditions or shortened life expectancy, which would make surgical options less desirable.
Several retrospective studies have compared the effectiveness of cyclophotocoagulation and glaucoma drainage implants for neovascular glaucoma; in these studies, glaucoma drainage implants have provided better control of IOP and preservation of vision than cyclophotocoagulation. Trabeculectomy without antimetabolites has not proven to be an effective choice for treating the disease, with failure rates up to 80 percent being reported. Adding MMC or 5-Fluorouracil has improved success rates, but neovascular glaucoma is still associated with success rates as low as 28 percent at five years after surgery, and encapsulated bleb formation.
For these reasons, glaucoma drainage implants are often chosen to reduce intractable elevated IOP in eyes with neovascular glaucoma. Unfortunately, the literature contains no direct comparisons between trabeculectomy and drainage device implantation, but given the poor track record of trabeculectomy with neovascular glaucoma, surgeons often opt for the drainage device.
Choosing a Drainage Device
Current drainage devices can be broadly categorized as either being designed to restrict aqueous flow through the use of a valve, or as non-flow-restrictive. Restricting flow is a strategy for avoiding early postop hypotony; the Ahmed valve uses this type of design.
The Baerveldt and Molteno implants fall into the other category; as a result, they require various special techniques to avoid early hypotony.
So far, attempts to determine whe-ther one specific implant produces better outcomes than the others (in terms of postoperative IOPs, number of medications and surgical success rates), when treating glaucoma in general, have been inconclusive. At the same time, studies have found little benefit from the addition of an antifibrosis regimen such as the use of mitomycin-C during implantation. (One study without a control group for comparison found that an intensive regimen of 5-fluorouracil and mitomycin C may improve the success rates of Ahmed glaucoma valve implantation somewhat,2 but the doses that would commonly be used during trabeculectomy appear to have little or no effect on the outcomes of surgery.3)
Another issue surrounding the use of glaucoma drainage implants is whether their efficacy is affected by the type of glaucoma being treated. We do know that uveitic glaucoma isn't associated with a higher drainage implant failure rate, especially when the uveitis is being controlled via immunomodulatory therapy;4 severe ocular surface disease doesn't appear to have a negative impact on success rates;5 and the location of the implant, age of the patient and previous surgery do not, for the most part, undermine the success rate of implant surgery.6
To help expand our understanding of how effective glaucoma drainage implants are in the treatment of NVG, we studied the Ahmed glaucoma valve in the treatment of this disease.7,8 We wanted to know whether the Ahmed glaucoma valve was effective for lowering IOP in these patients, and how its effectiveness with neovascular glaucoma compares to use in other glaucoma diagnoses. We were also interested to know whether patients lost vision, and if so, whether it was due to IOP control issues.
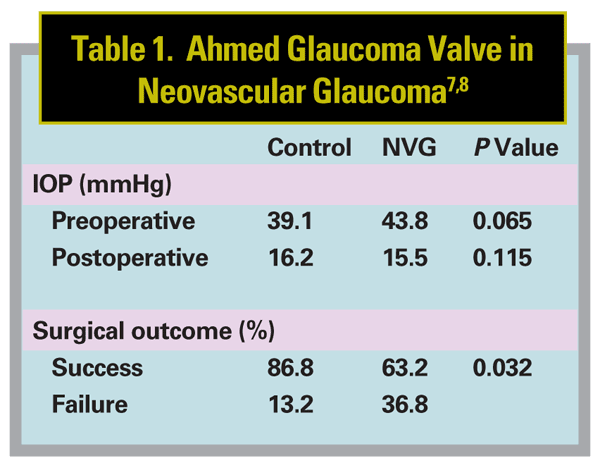
To answer these questions, we retrospectively reviewed data regarding 76 eyes of 76 patients; 38 were consecutive neovascular glaucoma patients that I had surgically treated with the Ahmed Glaucoma Valve. All surgeries were originally performed in response to elevated IOP that had failed to respond to previous medical therapy, laser treatment or glaucoma surgery. Controls were age- and sex-matched; they were statistically comparable in terms of race, presence of hypertension, lens status and preoperative IOP. The mean follow-up period was 18 months in control eyes and 17.4 months in neovascular glaucoma eyes. We defined surgical success as a postop IOP between 6 and 21 mmHg, with or without additional glaucoma medications, without further glaucoma surgery and without loss of light perception.
What Our Data Showed
At the most recent follow-up, both groups showed a significant decrease in mean IOP (p=0.001), and a significant decrease in number of medications needed. (See Table 1). No implants needed to be removed. How-ever, despite several similarities, a number of notable differences were found between the groups:
• Mean IOP was very similar: 16.2 ±5.2 mmHg in controls and 15.5 ±12.5 mmHg in neovascular glaucoma eyes (p=0.115).
• Mean number of glaucoma medications was lower in the neovascular glaucoma eyes: 1.5 ±1.4 in controls and 0.7 ±1.1 in neovascular glaucoma eyes (p=0.004). Both groups showed a sharp reduction of the number of medications during the immediate postoperative period, followed by a gradual increase during the first two to three years postop and a decreasing number of glaucoma medications thereafter.
• Mean postoperative IOP and number of medications during the follow-up period were similar in controls and neovascular glaucoma eyes.
• 44.7 percent of controls and 34.3 percent of neovascular glaucoma eyes developed a hypertensive phase within three months of Ahmed glaucoma valve implantation, which was not significantly different (p=0.348).
• Despite these similarities, the success rate was significantly lower in neovascular glaucoma patients (63.2 percent) than in controls (86.8 percent) (p=0.032).
• Fourteen neovascular glaucoma eyes (36.8 percent) met the definition of surgical failure; only five control eyes (13.2 percent) did. The causes differed: The majority of control eyes failed due to increased IOP, while neovascular glaucoma eyes were considered surgical failures due to hypotony and loss of light perception.
• At the most recent visit, visual acuity was improved or within one Snellen line in 86.8 percent of control eyes; this was only true in 60.5 percent of neovascular glaucoma eyes (p=0.018). (The outcomes achieved by the control group in this study were comparable to outcomes in other studies that evaluated the impact of Ahmed valve implantation on subjects with mixed glaucoma diagnoses.)
• Despite control of the IOP by the Ahmed Glaucoma Valve, diagnosis of neovascular glaucoma was associated with an increased risk of surgical failure.
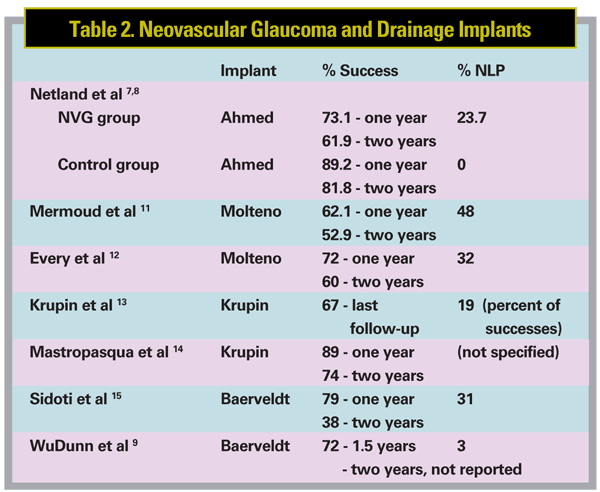
Would similar results be found if other drainage devices were chosen instead? Studies have been conducted using the Molteno, Krupin and Baerveldt implants;9-15 results from these studies are summarized in Table 2 (above). Like our study, they found that the devices did control IOP, and success rates were comparable to ours as well.
Notably, these studies found that pa-tients with neovascular glaucoma treated with glaucoma surgery may have difficulties with follow-up because of co-morbid conditions or death. In one study, the life expectancy of patients with neovascular glaucoma treated with Molteno implants was reduced by 52 percent.16 In another study, during the follow-up period after treatment with the Molteno implant, the death of 101 neovascular glaucoma patients out of 130 (78 percent) was reported.12 During the follow-up period after treatment with the Krupin implant, 17 of 79 patients (22 percent) died in one study,13 and 22 of 28 patients (79 percent) died in another study.14 We also experienced some dropout during follow-up in our study, but we did not analyze the reason for this.
It's important to note that these mortality rates are not related to the eye treatments or the drainage implants. They reflect the reality that this eye disease is associated with serious underlying medical problems, many of which can be life-threatening. In fact, patients are very glad to have their eyes treated with drainage devices, and their retinopathy treated (e.g., with laser and Avastin); their eyes are much more comfortable, and the majority of patients retain vision. Despite the high morbidity seen in these patients, without treatment all of them would develop blind, painful eyes, often requiring enucleation to relieve discomfort. So treatment definitely helps.
There's More to Be Done
One of the goals when treating neovascular glaucoma is to lower elevated pressure. It seems clear, based on our study and studies of other drainage implants, that implants can effectively lower IOP in patients with neovascular glaucoma. However, as our study showed, visual outcomes can still be poor. About one-quarter of those treated lose vision, and the success rates are significantly lower in eyes with neovascular glaucoma than in control eyes.
At the same time, the majority of treated patients do retain vision and experience relief of discomfort. In this regard, most patients are successfully treated. It's also true that the criteria for "failure of shunt surgery" are quite strict, and are not always related to whether the patients feel that the treatments they've undergone were worthwhile or not. I recall very few patients who did not feel they were improved or stabilized in some way after the eye surgery.
So, it appears that the Ahmed valve serves its intended purpose, lowering IOP to a more physiologic level; but that is clearly not sufficient to prevent further damage in these patients.
Although it's not possible to draw any firm conclusions about the reason for this, the fact that the majority of eyes with loss of light perception vision had IOP between 5 and 22 mmHg suggests that underlying disease may be a major causal factor. That finding suggests that a key area for future research should be the preservation of retinal and optic nerve function in these patients.
Dr. Netland is DuPont Guerry III Professor and Chair; UVA Depart-ment of Ophthalmology;
1. Sivak-Callcott JA, O'Day DM, Gass DM, Tsai JC. Evidence-based recommendations for the diagnosis and treatment of neovascular glaucoma. Ophthalmology 2001;108:1767-1778.
2. Alvarado JA, Hollander DA, Juster RP, Lee LC. Ahmed valve implantation with adjunctive mitomycin C and 5-fluorouracil: Long-term outcomes. Am J Ophthalmol 2008;146:276-284.
3. Costa VP, Azuara-Blanco A, Netland PA, Lesk MR, Arcieri ES. Efficacy and safety of adjunctive mitomycin C during Ahmed Glaucoma Valve implantation: A prospective randomized clinical trial. Ophthalmology 2004; 111:1071-1076.
4. DaMata A, Burk SE, Netland PA, Baltatzis S, Christen W, Foster CS. Management of uveitic glaucoma with Ahmed glaucoma valve implantation. Ophthalmology 1999;106:2168-2172.
5. Netland PA, Terada H, Dohlman CH. Glaucoma associated with keratoprosthesis. Ophthalmology 1998;105:751-757.
6. Rachmiel R, Trope GE, Buys YM, Flanagan JG, Chipman ML. Intermediate-term outcome and success of superior versus inferior Ahmed glaucoma valve implantation. J Glaucoma 2008;17:584-590.
7. Netland PA. The Ahmed Glaucoma Valve in neovascular glaucoma. Trans Am Ophthalmol Soc 2009; 107: 325-342.
8. Netland PA, Ishida K, Boyle JW. The Ahmed Glaucoma Valve in patients with and without neovascular glaucoma. J Glaucoma 2010 Feb 15. [Epub ahead of print]
9. WuDunn D, Phan A-D, Cantor LB, Lind JT, Cortes A, Wu B. Clinical experience with the Baerveldt 250-mm2 implant. Ophthalmology 2006;113:766-772.
10. Molteno AC, Van Rooyen MM, Bartholomew RS. Implants for draining neovascular glaucoma. Br J Ophthalmol 1977;61:120-125.
11. Mermoud A, Salmon JF, Alexander P, Straker C,
12. Every SG, Molteno ACB, Bevin TH, Herbison P. Long-term results of Molteno implant insertion in cases of neovascular glaucoma. Arch Ophthalmol 2006;124:355-360.
13. Krupin T, Kaufman P, Mandell AI, et al. Long-term results of valve implants in filtering surgery for eyes with neovascular glaucoma. Am J Ophthalmol 1983;95:775-782.
14. Mastropasqua L, Carpineto P, Ciancaglini M, Zuppardi E. Long-term results of Krupin-Denver valve implants in filtering surgery for neovascular glaucoma. Ophthalmologica 1996;210:203-206.
15. Sidoti PA, Dunphy TR, Baerveldt G, et al. Experience with the Baerveldt glaucoma implant in treating neovascular glaucoma. Ophthalmology 1995;102:1107-1118.
16. Blanc JP, Molteno AC, Fuller JR, Bevin TH, Herbison P. Life expectancy of patients with neovascular glaucoma drained by Molteno implants. Clin Experiment Ophthalmol 2004;32:360-363.



