As every cataract surgeon knows, getting an ideal refractive outcome when implanting an intraocular lens is a challenge. It’s still impossible to know for sure exactly where the implanted lens will end up sitting inside the eye; there are a limited number of options for correcting a poor refractive outcome (should it occur); and addressing presbyopia via a premium implant is far from a sure thing.
Not surprisingly, a number of new technologies that may offer solutions to these problems are in the pipeline. Here, three of the most promising are profiled by surgeons familiar with them.
Postop Refractive Adjustment
One of the most interesting new refractive options under development is the Perfect Lens (Perfect Lens LLC, Irvine, California). Despite its name, the product is not actually an intraocular lens; instead, it’s a system that’s capable of altering the refractive power of an IOL that’s already been implanted inside the eye, using a laser.
The laser system comprises a femtosecond laser and an optical focusing system. Once the focusing system is placed on the eye, the software is able to locate the implanted lens and then direct the laser to a 50-µm area inside the lens, where it makes whatever refractive adjustment is desired without having any disruptive effect on the optical characteristics of the lens, or the comfort or vision of the patient. (According to the company, the laser energy used is far less than the amount required for YAG procedures or cataract surgery.) The goal is to correct refractive errors remaining after the implantation of a lens, or to make other adjustments requested by the patient. Perhaps most remarkable, this technology appears to work on almost any implanted lens, from any manufacturer.
Y. Ralph Chu, MD, founder and medical director of Chu Vision Institute in Bloomington, Minnesota, and adjunct associate professor of ophthalmology at the University of Minnesota, is on the scientific advisory board for Perfect Lens. (He has no financial interest in the technology.) He notes that the version of the technology that he’s seen uses a femtosecond laser that’s similar to what today’s surgeons use. “The laser has a docking system similar to the cone-based system,” he says. “The patient lies down on a bed. There’s a unit that stabilizes the eye itself; then the laser docks to a ring, so the eye can be held steady during the procedure. It’s a very quick procedure that takes only a few seconds.
“It’s almost like creating a lens within a lens,” he continues. “The change doesn’t add any thickness or alter the shape of the overall lens; instead, it changes the hydrophilicity of the lens. The laser excites certain molecules inside the lens, causing the refractive index to change. The bottom line is, instead of cutting a flap and doing corneal surgery, you just change the power of the lens and you’re done. The changes take place inside the eye, so there’s no pain, no corneal dryness and no recovery period. Furthermore, it’s not specific to just one company’s lens. Right now they’re working with acrylic lenses, and so far, they can do this with any one of them, including lenses from J&J Vision, Bausch + Lomb, Zeiss and Alcon, among others. That’s why it’s so exciting.”
Dr. Chu says that Ruth Sahler, executive vice president and director of research and development at Perfect Lens, developed the optical pattern system that changes the refraction or dioptric power of the IOL with the assistance of Josef Bille, vice president at the company. “The system is very precise, down to 0.01 D,” Dr. Chu says. “At one point they took about 15 different IOLs and altered their refractions to make them 20 D. The lenses ended up being within 0.01 D of each other. Meanwhile, the process leaves the optical quality of the lens unchanged, so the modulation transfer function curves look excellent. There’s very little degradation.”
Dr. Chu notes that today, the focus in cataract surgery is on getting a precise outcome. “A big part of that has been managing the challenge of predicting where the lens will sit in the bag,” he points out. “That’s challenging because every eye is different. But with this technology, you can simply wait until the foundation for the lens is set and the IOL is settled, and then adjust the power to get the precise outcome you’re trying to achieve.”
Dr. Chu says he’s not aware of any limit to how much the refractive power can be changed. “The data so far suggests the system can make changes of 10 D or more,” he says. “You could basically put in a 20-D lens in every patient. If the patient needs a 24-D lens, you could make a 4-D adjustment—and it could be made in either direction, plus or minus.
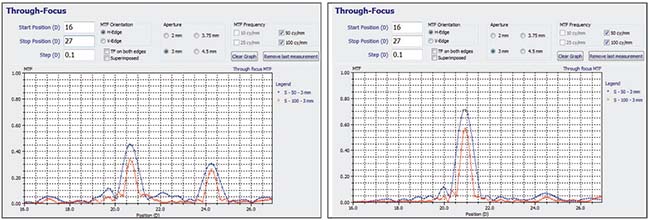 |
| The Perfect Lens laser system uses a femtosecond laser to make refractive adjustments to a previously implanted intraocular lens. So far, the process appears to work on lenses from any manufacturer. Changes appear to be extremely precise and can range up to 10 D, and include the ability to change a multifocal to a monofocal, or vice versa. The graphs above show the refractive profile of a multifocal IOL before (left) and after adjustment (right). Lenses can be altered multiple times, and the refractive changes appear to be stable. |
“What may be of particular interest to surgeons is that this technology can reverse multifocality,” he continues. “It can take a monofocal and make it multifocal, or take a multifocal lens and make it monofocal. (See example, above.) It’s like an eraser. This will be a boon to surgeons offering premium lenses such as multifocals because of the difficulty of predicting who will tolerate a multifocal lens. If the patient isn’t happy, we can eliminate the multifocality of the lens. It’s not an IOL exchange; we simply reverse it. Furthermore, I think this capability will be useful for every surgeon that wants to do refractive cataract surgery, even if you don’t offer premium IOLs. If a patient wants to try monovision using monofocal IOLs, you’ll be able to adjust one eye. If they don’t like the result, you can adjust it back.”
Dr. Chu adds that apart from limited space inside the IOL, there’s no apparent limit to how many times a lens can be changed or how far into the future changes can be made. “In the lab they’ve switched refractive power back and forth multiple times,” he says. “They’ve taken a multifocal and turned it into a monofocal; then back into a multifocal; then back into a monofocal. There’s no time limit that I’m aware of. There’s no leaching of material and no alteration in the adjusted refraction over time, from what they’ve seen in the lab in animals. It’s not like it wears off in a year and then you have to do it again. The lens is solid. You’re just making a change inside the lens, and it stays that way.”
One obvious question is how this technology differs from the Light Adjustable Lens (RxSight) which was recently approved by the U.S. Food and Drug Administration. Dr. Chu says he has no firsthand experience with the LAL, but notes there are significant differences between the two products. “Because of the LAL’s technology, the altered prescription is ‘locked in’ using ultraviolet light,” he notes. “That requires implanting their proprietary lens, then protecting your eyes from UV light with special glasses until the right moment, which could take weeks, and potentially coming back to the office multiple times to lock in the prescription. In contrast, the Perfect Lens technology doesn’t require the patient to wear protective glasses. You simply implant your lens of choice and give it four to six weeks to stabilize. Then, if the patient desires, you can perform—for lack of a better term—an enhancement to the IOL. The patient just goes about living life, and if you want to change the prescription next month, or six months from now, or 20 years from now, you can do it.”
Dr. Chu says the company is gearing up to begin human trials in the United States. “I believe that will start this year at the University of Utah,” he says. “At the moment the technology is still in the prototype phase, but things are moving forward quickly. I believe any obstacles we encounter will be surmountable, because we’re using two familiar technologies: femtosecond lasers and IOLs. Marrying the two is an exciting prospect.”
A Small-aperture IOL
Most ophthalmologists are familiar with the KAMRA corneal inlay from SightLife Surgical (originally developed by AcuFocus), which works by using the pinhole effect—aka small-aperture optics—to alleviate distortion and expand depth-of-field. AcuFocus has now created a monofocal intraocular lens that uses the same principle to achieve similar refractive results. The IC-8 IOL is a 6-mm-diameter one-piece hydrophobic acrylic lens that incorporates a 3.23-mm doughnut-shaped opaque mask with a central 1.36-mm hole through which light can pass. (See picture, right.) The lens is available in powers of 15.5 to 27.5 D in 0.5-D steps. It’s implanted via a single-use injector through a 3.2- to 3.5-mm incision.
 |
| The IC-8 intraocular lens from AcuFocus uses the pinhole effect to extend depth-of-focus dramatically and compensate for factors such as astigmatism, corneal aberrations and failure to hit the intended post-surgical refractive target. |
“The IC-8 IOL is an aspheric monofocal IOL that has an opaque mini-ring embedded in it to extend depth-of-focus from near to far without gaps in vision,” says Dr. H. Burkhard Dick, chairman of the University Eye Hospital in Bochum, Germany, and lead investigator of the clinical trials involving the IC-8 IOL. “The lens design is based on the well-established small-aperture principle; it works by allowing only the central, focused light to reach the retina, removing the blur caused by peripheral defocused light. This results in the highest quality of vision over the broadest continuous range of any premium IOL currently available. It provides up to 3 D of range-of-vision with excellent visual quality, providing relief from presbyopia. Currently, the IC-8 IOL is implanted monocularly, usually in the nondominant eye. Binocular implantation has been done in a small cohort of patients, and work is being done to optimize this option for surgeons and patients.”
Dr. Dick says a recent multicenter study of the small-aperture IOL showed excellent visual performance, safety, patient satisfaction and tolerance to residual astigmatism six months after implantation. “At six months, patients achieved, on average, 20/16 for distance, 20/20 for intermediate and 20/25 for near uncorrected visual acuity,” he says. “Outcomes can be further optimized by achieving the refractive targets of -0.75 D in the IC-8 IOL eye and plano in the companion eye. Ninety-six percent of the patients said they would have the surgery again.”1
Dr. Dick points out that a lens based on the small-aperture principle may have several advantages over multifocal IOLs. “First, the IC-8 produces high quality, full-range vision without blurry zones,” he says. “Second, the lens is very tolerant of refractive error misses. A deviation of more than 0.5 D from the intended refractive target will result in a loss of one or two lines of vision with a typical monofocal or multifocal IOL; the IC-8 IOL will provide a reliable range of vision with as much as a 1-D deviation from the intended refractive target. In clinical studies, 100 percent of patients receiving the IC-8 IOL maintained 20/40 or better UDVA, even with a [postoperative] refractive error ranging from 0.50 to -1.50 D.2 Third, if the eye has an irregular cornea, multifocality can result in poor quality of vision and severe photic phenomena, among other issues. The small-aperture design of the IC-8 IOL eliminates peripheral defocus and aberrated light, resulting in improved quality and range of vision.”
Dr. Dick adds another important benefit of the small-aperture optics; the IC-8 compensates for up to
1.25 D of corneal astigmatism, with no effect on visual acuity. “This means the 82 percent of patients who present for cataract surgery with less than 1.5 D of corneal astigmatism can enjoy the benefit of astigmatism correction without the risks associated with toric IOLs,” he says. “Furthermore, if astigmatism is induced during surgery, the effects are mitigated by the small-aperture optics. This also simplifies the surgical process for the surgeon, as there is no IOL axis to align and, as a result, no marking or intraoperative alignment equipment needed to implant the lens. Additionally, post-implantation IOL rotation has no influence on the efficacy of the IC-8 lens.”
A common concern with small-aperture optics is the possibility of consequences caused by the reduced amount of light reaching the retina. “Although the retina receives less light due to the elimination of peripheral rays, binocular contrast sensitivity is equivalent to that of a monofocal IOL,” Dr. Dick says. “At the 2017 European Society of Cataract and Refractive Surgeons meeting, Pablo Artal, PhD, reported that patients treated with a small-aperture implant over time adapt to the reduced light and actually perceive there to be between 30 and 60 percent more light than what is actually reaching the retina. So, after a period of adaptation, patients are unlikely to notice the reduced light, unless they compare the IC-8 eye directly to the fellow eye. Other research has shown that, over time, neuroadaptation produces a stereoacuity effect similar to normal binocular vision, wherein the dominant eye negates poor visual performance resulting from low lighting.3,4 Patients of any age, regardless of their work-lighting conditions, can be considered as candidates for the IC-8 implant.”
Asked whether the embedded ring might interfere with postoperative retinal exams, Dr. Dick says the impact is minimal. “Despite the posterior positioning of the IC-8 IOL, fundus photography and angiography are entirely possible,” he says. “Physicians treating patients with the early small-aperture IOL implants report few differences in retinal imaging from the fellow eye implanted with a monofocal IOL. I myself have performed retinal surgeries including membrane peel, vitrectomy with indentation, air fluid exchange, cryotherapy and retinal lasering with success. The presence of the mask does require surgeons to modify their technique, but visualization and stereopsis are still good and procedures can be performed without incident.”
The Omega Gemini Capsule
Another unique development relating to intraocular lenses is the Gemini Refractive Capsule, from Omega. The Gemini Refractive Capsule is a three-dimensional device designed to be implanted inside the capsular bag, holding the space open and allowing controlled placement of a refractive lens—and potentially other items—at a known distance relative to the front and back of the eye.
The company notes that traditional cataract surgery has an inherent “Achilles heel:” A standard IOL is about one-fifth the thickness of the cataractous lens that’s being removed. That makes it difficult or impossible to determine the final position of the lens implant inside the eye. This device is intended to eliminate that concern, while simultaneously creating the possibility of other uses for the intracapsular space. (The company notes that the device is associated with more than 40 patents issued and pending in the United States and around the world.)
“This device is designed to keep the capsular bag open, and it allows for modularity,” explains John Berdahl, MD, a partner at Vance Thompson Vision in Sioux Falls, South Dakota, and associate professor at the University of South Dakota. (Dr. Berdahl is a consultant to Omega.) “It contains tiny shelves designed to allow placement of an IOL in a particular position relative to the cornea. The idea is that because you’ll know exactly where the Gemini Capsule is, you’ll know exactly what the position of the lens is. Furthermore, you can later move the lens to a different shelf if you need to. That should result in fewer postoperative enhancements, as well as letting the surgeon alter the refraction in the future as the eye ages, even if you nailed the refraction in the original surgery. In addition, the device is designed to work with intraoperative aberrometry, so those kind of adjustments could be made while the patient is on the operating table.
“The extra shelves could also be used to add other inserts,” he continues. “One
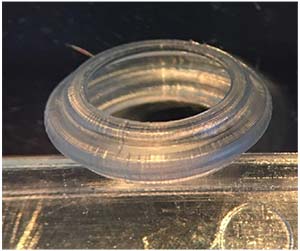 |
| The Gemini Refractive Capsule from Omega is a three-dimensional device designed to be implanted inside the capsular bag, holding the space open and allowing controlled placement of a refractive lens at a known distance relative to the front and back of the eye. Other items such as sensors and drug-delivery devices could also be placed in the Capsule. |
possibility would be an extra lens to alter the refractive result, whether that involves simply adjusting the refraction or adding some new multifocal technology that comes along in the future. Sensors of different types could also be placed inside the capsule. That area is important real estate that can allow us to gain a lot of insights into how the eye is functioning and how the body is functioning. Drug-delivery devices could be placed there as well.”
Obviously, the device is larger than a typical intraocular lens. “We definitely want this to be able to go through a small incision,” notes Dr. Berdahl. “That’s part of the reason the device is modular; the two separate, flexible parts of the device will be inserted in two steps so you don’t have to put the full volume of the device through the incision all at once.”
Given the size of the device, might it obscure the surgeon’s view of the retinal periphery when it’s in position? “I don’t think that will be the case,” Dr. Berdahl says. “Even with a lens like the AcuFocus IC-8, you’re still able to see light from the peripheral retina because the lens is located near the nodal point. Furthermore, the capsule opacifies when it contracts around a traditional IOL, but that doesn’t obstruct the surgeon’s view. So any opacity that’s created by the peripheral frame of the Gemini Capsule is unlikely to affect our ability to see the peripheral retina.”
Asked if he’s aware of any drawbacks to this technology, Dr. Berdahl says it’s too early to know if any will arise. (The device has been implanted in humans, but the company hasn’t initiated U.S. Food and Drug Administration trials yet.) “Once we evaluate the implant in humans and see how predictable it is and how well it responds, we’ll know more,” he says. “The principles behind it are sound, but we always learn things when we go into clinical trials. We’re excited to see what those data show.” The company notes that the design incorporates tried-and-true optical principles and materials, which should minimize obstacles to FDA approval.
Dr. Berdahl believes the future of cataract surgery and IOLs will be a conversation about the adjustability of lenses, rather than their upgradability and exchangeability. “It’s an exciting time for IOLs because we’re doing things to them after they’re placed in the eye, in order to get better outcomes,” he says. “I think that’s where things are heading.” REVIEW
1. Dick H, Piovella M, Vukich J, Vilupuru S, Lin L. Prospective multicenter trial of a small aperture intraocular lens in cataract surgery. J Cataract Refract Surg 2017;43:7:956-968.
2. Paley GL, Chuck RS, Tsai LM. Corneal-based surgical presbyopic therapies and their application in pseudophakic patients. J Ophthalmol 2016;2016:5263870.
3. Fernandez EJ, Schwarz C, Prieto PM, Manzanera S, Artal P. Impact on stereo-acuity of two presbyopia correction approaches: Monovision and small aperture inlay. Biomed Opt Express 2013; 4:822-830.
4. Schwarz C, Manzanera S, Artal P. Binocular visual performance with aberration correction as a function of light level. J Vision 2014;14;14;6.