Presentation
A 47-year-old man presented to Wills Eye Oculoplastic Department for a second opinion and evaluation of fullness of the left eye. He reported that he had an outpatient computed tomography scan and magnetic resonance imaging scan (Figure 1) which were notable for a “large left orbital mass, primarily intraconal with extraconal extension superiorly and laterally, concerning for a benign, slow-flow venous malformation.”
Upon initial ocular examination the patient had a visual acuity of 20/30 by pinhole in the right eye and 20/20 in the left eye. No afferent pupillary defect was noted. Confrontational visual fields were full. Decreased abduction of the left eye was noted, with subjective diplopia in left gaze. Intraocular pressures were 18 mmHg in both eyes. Hertel measurements showed 5 mm of proptosis of the left eye. There was noted to be some resistance to retropulsion in the left eye, with superotemporal fullness. On dilated fundus exam he was noted to have choroidal folds in the left eye. He was referred to neurosurgery at Jefferson for consideration of embolization of the left vascular orbital mass.
Due to the COVID-19 pandemic, the patient was initially seen by neurosurgery via a telemedicine appointment. At the time of his neurosurgical follow up, the patient reported no blurry vision, double vision, pain or other visual/ocular symptoms, other than intermittent fullness in the left eye. The patient was scheduled for a diagnostic cerebral angiogram to determine whether this was a high-flow vascular lesion and whether he would be a good candidate for embolization. This was notable for no obvious vascular lesions within the orbit, confirming no high flow to the lesion. In consultation, ophthalmology and neurosurgery determined that direct puncture onyx embolization followed by orbitotomy was indicated.
Medical History
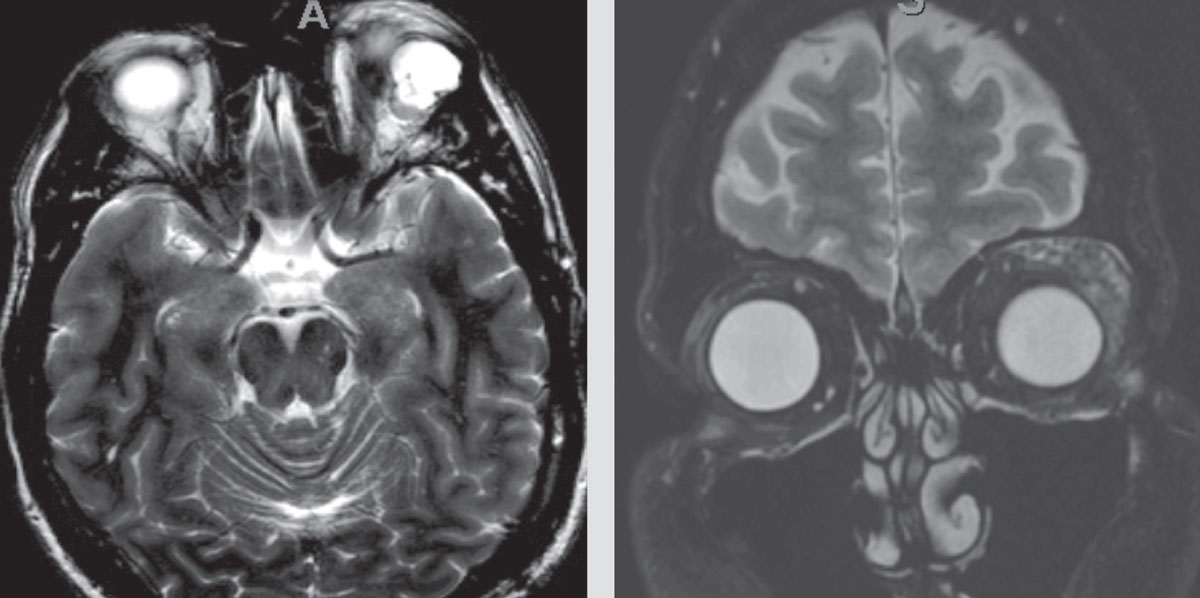 |
| Figure 1. Outside imaging center MRI images with left orbital mass, read as, “lobulated with enhancement of septations. A small vascular channel is seen … The most common lesion of this nature in this area is a slow-flow venous malformation. Other vascular lesions are not excluded.” |
The patient denied any past ocular history. Past medical history included testosterone supplementation for low testosterone levels. Family history included myocardial infarction on his paternal side and an unknown cancer on his maternal side. Social history was significant for tobacco use for a few decades, though the patient had quit seven years prior to presentation. Current medications included testosterone and vitamin D.
Exam
On postoperative day zero of his embolization, ocular examination demonstrated visual acuity of 20/20 in the right eye and 20/70 in the left eye. Pupils were noted to be 3 mm in dark/2 mm in light in the right eye and 4 mm in dark/3 mm in light in the left eye. There was no frank APD. Intraocular pressures were 14 and 22 mmHg in the right and left eyes, respectively. Extraocular motility was full in the right eye, with -0.5 adduction, -1 abduction, and -2 supraduction in the left. Moderate left upper lid edema and mild left lower lid edema were noted.
What is your diagnosis? What further workup would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
Oculoplastics proceeded with left orbitotomy under general anesthesia the following day. Intraoperatively, the lesion was found to be multiloculated, with many pockets of white cloudy fluid and other pockets of thick white toothpaste-like material. These pockets also contained onyx. The lesion didn’t appear to be avascular intraoperatively. The mass displaced the globe medially, inferiorly and anteriorly, with additional displacement of the left superior and lateral rectus muscles. The lesion was adherent to the lateral rectus, optic nerve and globe, and was impossible to resect completely. Partial mass resection and biopsy were completed.
Biopsy of the lesion resulted in a pathologic diagnosis of invasive, high-grade adenocarcinoma of the left lacrimal gland. Molecular evaluation revealed it was estrogen receptor negative, progesterone receptor negative, HER2 Neu positive. The patient was referred to medical oncology. His adenocarcinoma was found to strongly express the androgen receptor and his testosterone supplementation was discontinued. There was no evidence of metastatic disease. Otolaryngology performed a sentinel lymph node biopsy, which was negative. Repeat MRI, as expected, showed the mass to be smaller, but there was still residual disease in the orbit (Figure 2). The patient underwent left orbital exenteration and adjuvant radiation therapy.
Discussion
Primary ductal adenocarcinoma of the lacrimal gland is a rare malignancy that was first reported in 1996.1 The literature on ductal adenocarcinoma of the lacrimal gland is largely composed of case reports and reviews. Dr. Steffen Heegaard of the University of Copenhagen published a review and several case reports in 2017 in Acta Ophthalmologica, which described a 4:1 male-to-female predominance of patients in the literature, with a median age of 64 years at diagnosis.2
The most typical presenting symptoms have been reported to be proptosis and restriction of eye movements, with or without diplopia.3 Radiographically, it can present as a lobulated mass on CT. On MRI, they have been reported to appear similarl to salivary duct carcinomas, which have been described as having low signaling intensity on T-2 weighted images, ill-defined borders and invasion into surrounding structures.4,5 These ambiguous radiographical findings, along with the disease’s rarity, can lead to initial diagnostic uncertainty, as was the case for our patient.
While the literature on this rare primary malignancy is scarce, management and treatment decisions have been assisted by the pathological and molecular similarities to ductal carcinomas of the salivary gland and the breast. These neoplasms all originate from the epithelial lining of glandular ducts. Salivary duct carcinoma has been noted to be particularly histologically and genotypically similar to lacrimal duct adenocarcinoma.2,3,6 The demographics of salivary ductal carcinoma patients in the literature are also similar to those of lacrimal ductal adenocarcinoma, with a male predominance (71 percent) and a median age of onset of 66.7
The mainstay treatment of lacrimal gland ductal adenocarcinoma is staging followed by local resection, with consideration of adjuvant radiation therapy. Presentation with metastatic disease is reportedly unusual, only representing five percent of cases.2 However, eventual development of metastatic disease is not uncommon, with one recent literature review finding 58 percent of patients eventually had metastatic lesions.3 Another review found that half of patients presenting without metastasis that continued to follow up developed distant metastasis, and 41 percent of patients died of their disease.2
In addition to phenotypic and histological similarities to salivary duct carcinoma, there are genetic commonalities. Both neoplasms frequently have an amplification of human epidermal growth factor 2 (HER2).8,9 This amplification can be targeted by a monoclonal antibody, trastuzumab. This treatment results in survival benefit in HER2-positive ductal carcinoma of the breast. However, there is insufficient data on the use of trastuzumab in HER2-positive salivary duct carcinoma or lacrimal gland adenocarcinoma, and the limited data that’s been collected has not showed a significant effect on HER2-positive salivary duct carcinomas.10 Very limited data has showed that androgen deprivation therapy might be beneficial for patients with widely metastatic or recurrent disease.3
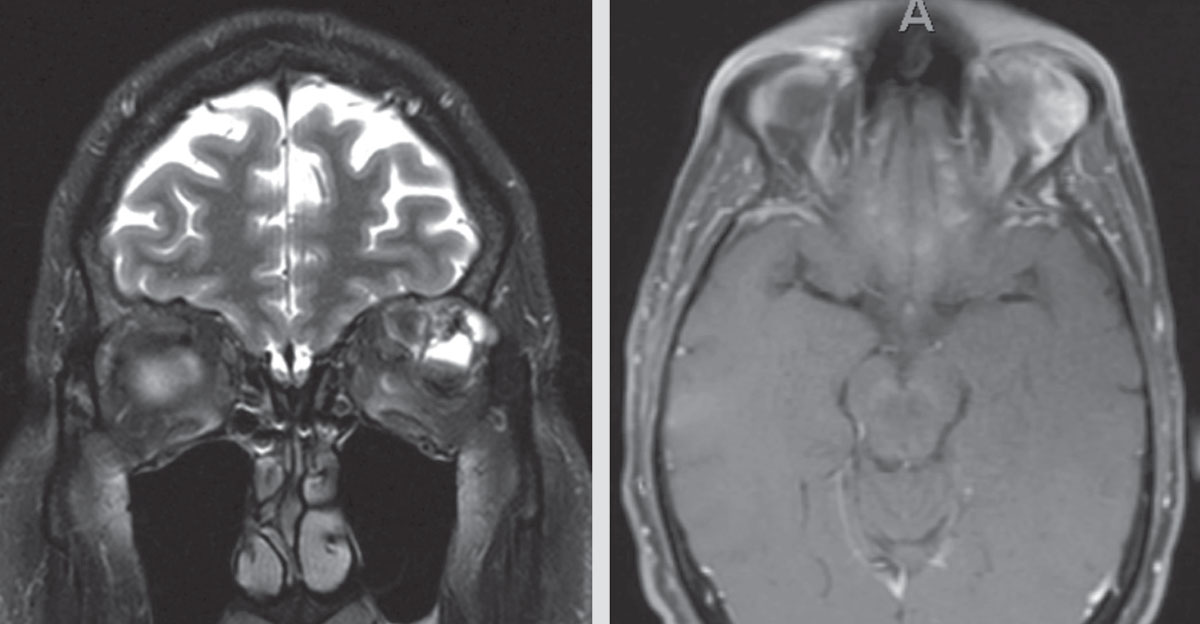 |
| Figure 2. Two MRI images demonstrating recurrent/residual disease in the left orbit months after the initial left orbitotomy. |
In conclusion, primary lacrimal ductal adenocarcinoma is a rare malignancy that typically presents with unilateral proptosis and limitation of extraocular movements. Primary lacrimal ductal adenocarcinomas appear to have a predilection for older men. They’re not typically metastatic at the time of presentation, but many patients develop distant metastasis and eventually die of their disease. Due to the rarity of this malignancy, treatment decisions are often informed by the phenotypic and genotypic similarities to salivary gland (and breast) carcinoma. Surgical resection followed by adjuvant radiotherapy is the mainstay of treatment, with exenteration often being necessary. Other treatment modalities, including adjuvant chemotherapy, adjuvant radiation, and/or therapies targeting specific mutations, are dependent on the presence or absence of metastasis, positron-emission tomography/computed tomography findings and histopathologic/genotypic findings.
This patient’s clinical presentation was initially concerning for either fungal interface keratitis or non-infectious interface inflammation. The decision to treat the patient aggressively with antifungal therapy or to treat the interface haze as a non-infectious inflammatory process with topical corticosteroids was based solely on clinical evaluation and interpretation of AS-OCT. The interface haze seen in this case corresponded to a diffuse hyperreflective process along the graft interface as seen in Figures 1 and 2. In contrast, fungal interface keratitis presents with characteristic focal infiltrates and an otherwise non-inflamed interface on AS-OCT,6 and this patient’s AS-OCT was therefore not consistent with a fungal interface keratitis. The interface in this case was more consistent with previously described elongated textural interface opacities.7 While difficult to differentiate fungal infection from other interface inflammation based on slit lamp examination alone, AS-OCT was critical in suggesting that this inflammation wasn’t fungal in etiology and could therefore be treated with frequent topical corticosteroids, albeit with close follow up. The improvement seen in the graft interface haze during treatment with topical corticosteroids supports the diagnosis of an inflammatory, rather than fungal infectious, etiology. Interestingly, the hyperopic astigmatism the patient manifested may be due to posterior corneal changes induced by the haze over time.
AS-OCT is a relatively new imaging modality that is an excellent tool for evaluating endothelial graft morphology and function after routine endothelial keratoplasty.8 This report highlights another potential use of AS-OCT to manage postoperative complications after endothelial keratoplasty, including diagnosing (or ruling out) fungal interface keratitis. As we begin to perform AS-OCT more routinely on eyes after endothelial keratoplasty, we hope to further characterize the spectrum of graft interface changes and use this knowledge to improve patient outcomes.
1. Katz SE, Rootman J, Dolman PJ, White VA, Berean KW. Primary ductal adenocarcinoma of the lacrimal gland. Ophthalmology 1996;103:157–62.
2. Andreasen S, Grauslund M, Heegaard S. Lacrimal gland ductal carcinomas: Clinical, morphological and genetic characterization and implications for targeted treatment. Acta Ophthalmol 2017;95:3:299-306.
3. Yang HY, Wu CH, Tsai CC, Yu WK, Kao SC, Liu CJ. Primary ductal adenocarcinoma of lacrimal gland: Two case reports and review of the literature. Taiwan J Ophthalmol 2018;8:1:42-48.
4. Kashiwagi N, Takashima S, Tomita Y, Araki Y, Yoshino K, Taniguchi S, et al. Salivary duct carcinoma of the parotid gland: Clinical and MR features in six patients. Br J Radiol 2009;82:800–4.
5. Motoori K, Iida Y, Nagai Y, Yamamoto S, Ueda T, Funatsu H, et al. MR Imaging of salivary duct carcinoma. Am J Neuroradiol 2005;26:1201–6.
6. Min KW, Park HK, Kim WY, Kim WS, Lim SD, Han HS, Hwang TS. Primary ductal adenocarcinoma of the lacrimal gland, associated with abundant intracytoplasmic lumens containing some eosinophilic hyaline globules: Cytological, histological and ultrastructural findings. Ultrastruct Pathol 2014;38:5:363-6.
7. Gilbert MR, Sharma A, Schmitt NC, Johnson JT, Ferris RL, Duvvuri U & Kim S. A 20-year review of 75 cases of salivary duct carcinoma. JAMA Otolaryngol Neck Surg 2016;142:489–495.
8. Dennie T. Metastatic, HER-2 amplified lacrimal gland carcinoma with response to lapatinib treatment. Case Rep Oncol Med 2015:1–3.
9. Skalova A, Starek I, Kucerova V, Szepe P & Plank L. Salivary duct carcinoma—A highly aggressive salivary gland tumor with HER-2/neu oncoprotein overexpression. Pathol Res Pract 2001;197:621–626.
10. Egebjerg K, Harwood CD, Woller NC, Kristensen CA, Mau-Sørensen M. HER2 positivity in histological subtypes of salivary gland carcinoma: A systematic review and meta-analysis. Front Oncol 2021;11:693394.



