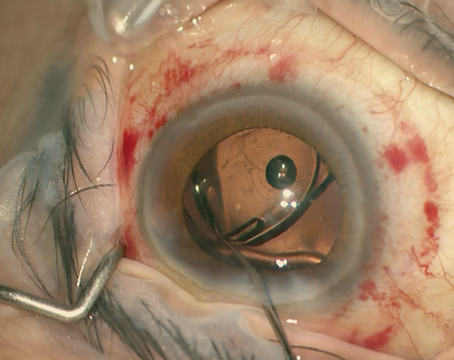
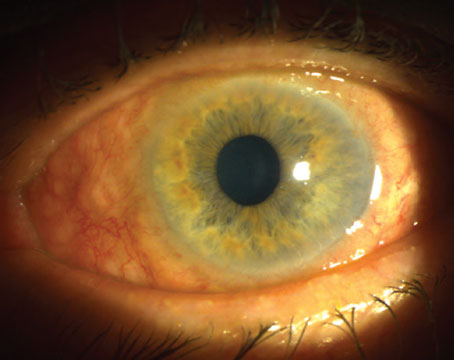
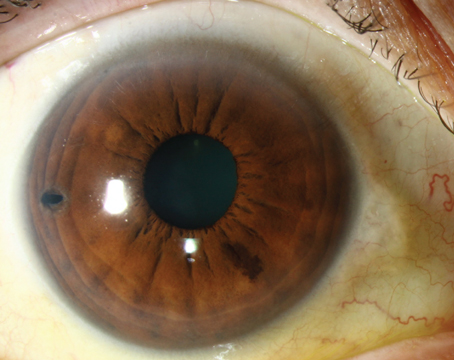
The initial differential for postoperative hypotony is broad, and includes inflammation, uveitis, wound leak or inadvertent bleb formation, cyclodialysis clefts, ciliochoroidal or retinal detachments, and cyclitic membrane. Broadly, the pathophysiology underlying hypotony can be divided into mechanisms of decreased aqueous production, increased aqueous outflow or a combination of the two. Easily investigable causes such as intraocular inflammation, wound leak and bleb formation were ruled out with the patient’s initial slit lamp examination. Furthermore, the initial funduscopic exam failed to reveal choroidal or retinal detachments.
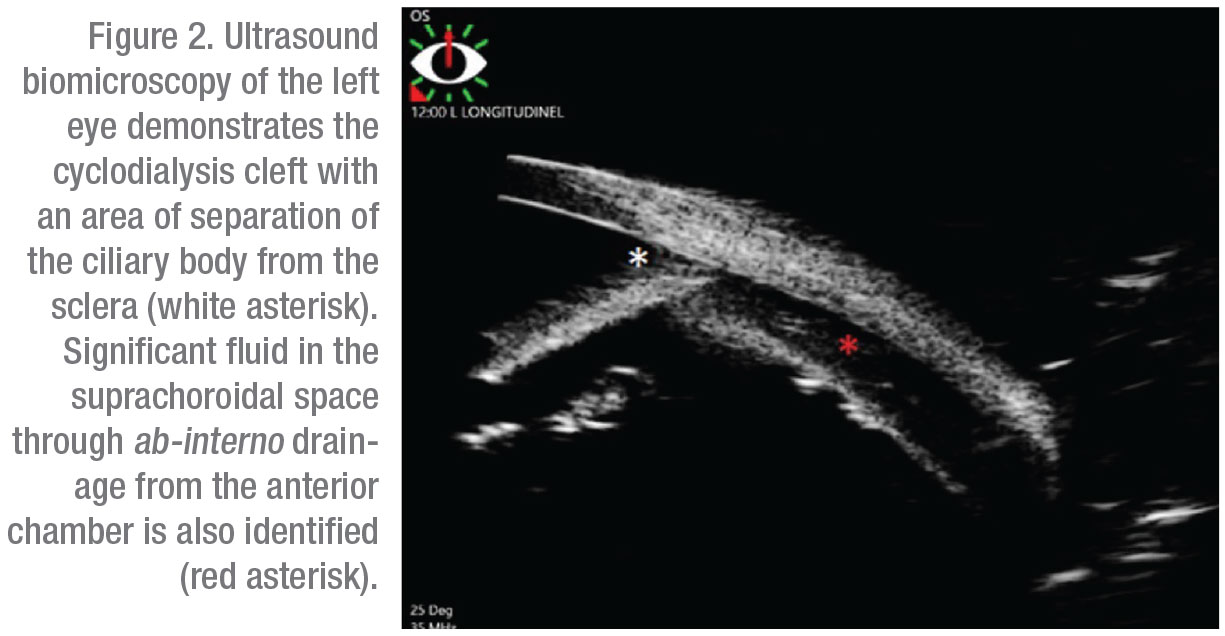
Gonioscopy revealed a nasal cyclodialysis cleft in the left eye between 9:45 and 11:30. Angles were otherwise found to be open to D40f2+ OD and (C)D35f2+ OS as graded by the Spaeth gonioscopy grading system. The extent of the cleft and associated suprachoroidal effusion was confirmed with ultrasound biomicroscopy (Figure 2). The patient was initially started on atropine drops two times daily for six weeks without improvement. This was followed by three sessions of argon laser treatments to the cyclodialysis cleft. However, vision and IOP remained unchanged and repeat gonioscopy confirmed persistence of the cleft.
Given the failure of conservative treatment options, an ab-externo cyclodialysis cleft closure was attempted through a partial thickness limbal scleral flap. Surgery was performed without complications, but postoperative gonioscopy revealed only partial closure with a persistent cleft between 8:30 and 9:30. Cycloplegics were restarted, but the cleft failed to resolve after observation for nine weeks and no improvement in IOP or vision was noted.
An ab-interno cyclopexy was then attempted. On postop day 1, IOP rose to 36 mmHg and slit lamp examination demonstrated patchy corneal microcystic edema and a deep anterior chamber with trace cells and flare. Gonioscopy subsequently confirmed closure of the cyclodialysis cleft. The IOP lowered with topical medications. Two weeks postoperatively, the patient presented for follow-up with worsening headaches and an IOP of 59 mmHg despite therapy with dorzolamide/timolol and netarsudil. Maximal medical therapy was initiated, but IOP remained elevated to 30 mmHg nearly a month later with little improvement in his persistent choroidal folds. He underwent Baerveldt tube shunt placement and his IOP subsequently improved to 12 mmHg. On his most recent follow-ups, vision OS had improved to 20/100 and IOP was stable between 11 and 12 mmHg.
 |
Discussion
Cyclodialysis clefts are a rare sequela of accidental or iatrogenic trauma, characterized by a separation of the ciliary body from its attachment to the scleral spur.1 Although previously observed only as a result of blunt trauma to the globe, a cyclodialysis cleft may also occur as a complication of any anterior segment surgery, particularly cataract surgery, goniotomy and iridectomy.2 Clefts provide an alternate outflow path for aqueous humor to the suprachoroidal space, which can result in chronic hypotony with potentially irreversible morphologic changes to the globe.1,3 However, as illustrated in this case, hypotony is not always symptomatic, and no pressure cutoff necessitates intervention in the absence of clinical signs of hypotony maculopathy. Generally, these patients present with visual decline from secondary changes in the structure of the eye including corneal edema and with-the-rule astigmatism, optic disc edema, maculopathy, choroidal folds or ciliochoroidal detachments.4,5
Clefts may be difficult to identify on gonioscopy, particularly in patients with a shallow anterior chamber or corneal edema. Intracameral injections of viscoelastic may be used to reform the AC and improve visualization of the angle. In some patients with shallow chambers, indentation gonioscopy may help detect the cleft. Although dynamic gonioscopy remains the gold standard in diagnosis, imaging with anterior segment OCT or ultrasound biomicroscopy may be valuable in patients in whom the view of the angle is compromised.4,6 These imaging modalities can delineate even small cyclodialysis clefts, and UBM in particular boasts excellent sensitivity for clefts that may be missed on gonioscopy without AC reformation.6
 |
Cyclodialysis clefts may be managed with medical, laser or surgical techniques, with the choice of therapy titrated to clinical severity. Smaller clefts, often defined as fewer than four clock hours, may resolve with medical cycloplegia.1 Cycloplegics such as atropine 1% help dilate the ciliary body ring and promote apposition of the detached muscle to the sclera.7 These drugs may be tried for six to eight weeks while tapering or discontinuing steroids to promote adhesion and fibrosis.1,7 Patients who fail medical management may be candidates for noninvasive laser techniques that promote local inflammation within the cleft. Argon laser,8 diode endophotocoagulation, trans-scleral diode9,10 and Nd:YAG laser have been employed, but success rates are modest and definitive closure with laser alone is reported to occur in less than 20 percent of cases.1,7
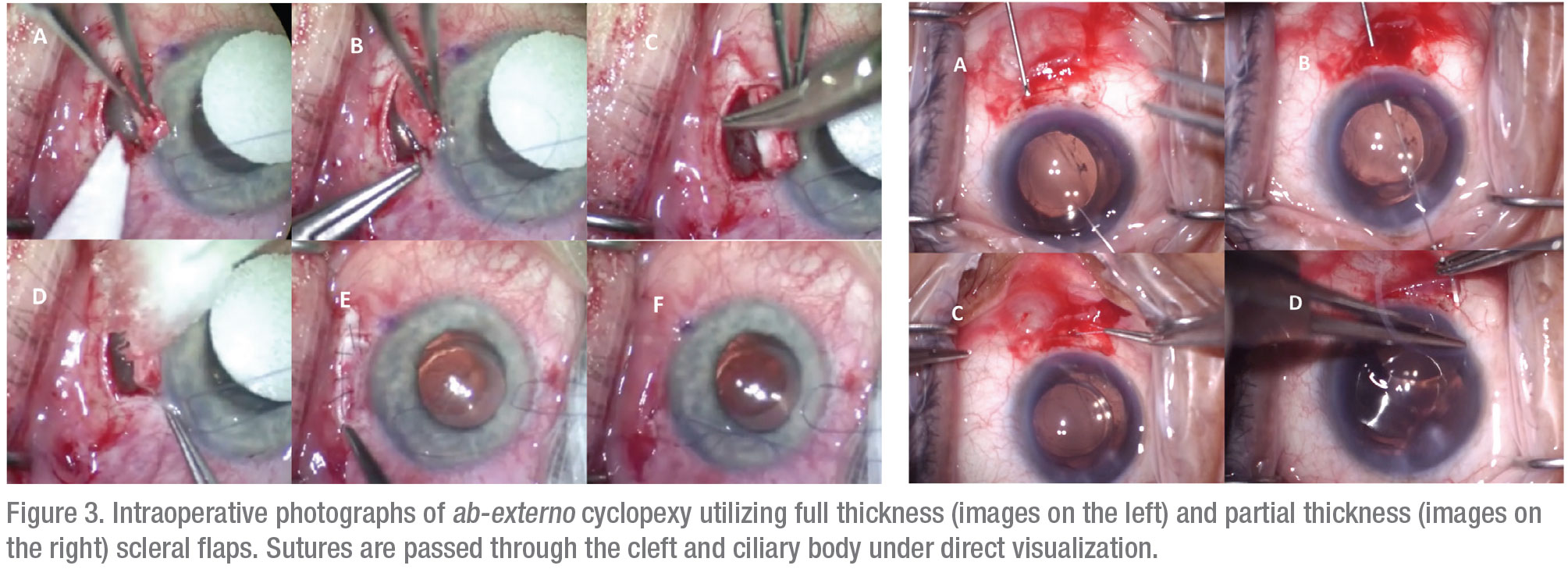
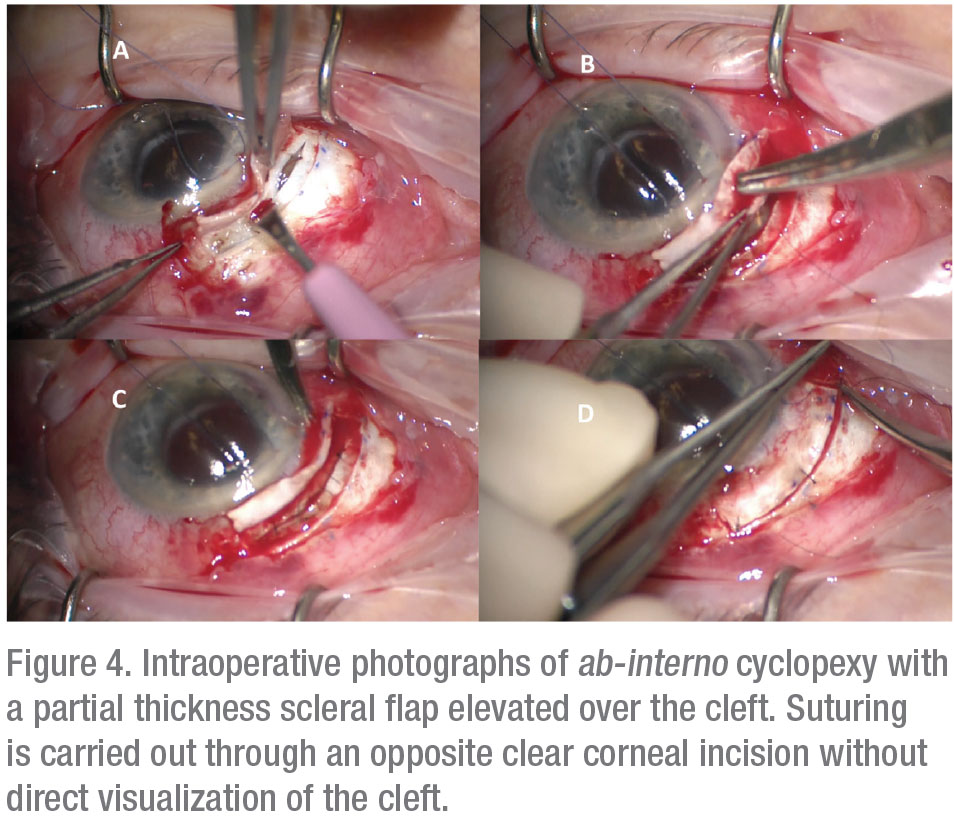
For patients who fail conservative management, fixation of the detached ciliary body to the scleral bed, known as cyclopexy, is the definitive treatment.11 A large multicenter series reported closure rates of greater than 96 percent for cyclopexy, although a lack of standardization in surgical nomenclature renders comparison of different surgical methods difficult.1,4 Traditional suture fixation may be performed through external (ab externo) or internal (ab interno) approaches. The ab-externo approach may be performed through either full or partial-thickness scleral flaps with direct visualization and suturing of the detached ciliary body (Figure 3). Various authors have described excellent surgical outcomes with variations of this technique, although complications such as hemorrhage, hypotony, retinal detachment and endophthalmitis have also been reported.2,5 Ab-interno approaches avoid the need for a full-thickness scleral cut-down and instead use sutures carried out from an opposite clear corneal incision (Figure 4).2,3
Surgical methods that employ mechanical tamponade of the ciliary body through either internal or external approaches have also been described. Internal tamponade may be successfully performed with the haptics of a sulcus intraocular lens or capsular tension ring (e.g., the Cionni ring).2 These devices may have more predictable outcomes than direct suturing and may be superior for extensive clefts; however, they also carry risks of ciliary body injury, erosion, pain and hemorrhage. External tamponade with anterior scleral buckling and silicone tubes and sponges offer less-reliable outcomes and may induce astigmatism, intolerable foreign body sensation or exposure of the implant.1–4 Pneumocyclopexy using intravitreal SF6 or C2F6 gas has also been explored, but is less-commonly used given the necessity for coordination between anterior and posterior segment surgeons.3,12
Postoperatively, successful closure of a cleft may be marked by an IOP spike that requires close monitoring and possible resumption of ocular hypotensive medications.2,13 These spikes are attributed to trabecular dysfunction and Schlemm’s canal collapse in the setting of the alternate aqueous outflow path. In our patient, even after resumption of topical glaucoma therapy, a Baerveldt tube shunt was eventually required to return IOP to the target range. Although long-standing maculopathy may not be reversible due to chronic fibrosis and distortion of the retina and choroid, most patients, including our own, demonstrate improvement in vision after reversal of low IOP.3,13
 |
Although minimally-invasive glaucoma surgery is gaining popularity in patients with mild or moderate glaucoma, these procedures—indeed, any anterior segment surgery—may result in iatrogenic damage to angle structures and creation of cyclodialysis clefts. Despite a growing body of literature regarding management, considerable ambiguity remains regarding the ideal timing and method of treatment. Conservative approaches with cycloplegics and laser are appropriate in the absence of visual changes. However, clinical evidence of hypotony should be addressed with cyclopexy in an efficient timeframe to minimize risk of permanent structural damage to the eye. Further investigation and long-term studies are still needed to evaluate the myriad surgical techniques for cleft closure, with the eventual goal of creating well-defined treatment algorithms for this rare complication. REVIEW
1. Popovic M, Shareef S, Mura JJ, et al. Cyclodialysis cleft repair: A multi-centred, retrospective case series. Clin Exp Ophthalmol 2019;47:2:201-211.
2. González-Martín-Moro J, Contreras-Martín I, Muñoz-Negrete FJ, Gómez-Sanz F, Zarallo-Gallardo J. Cyclodialysis: An update. Int Ophthalmol 2017;37:2:441-457.
3. Ioannidis AS, Barton K. Cyclodialysis cleft: Causes and repair. Curr Opin Ophthalmol 2010;21:2:150-154.
4. Selvan H, Gupta V, Gupta S. Cyclodialysis: An updated approach to surgical strategies. Acta Ophthalmol 2019;97:8:744-751.
5. Ormerod LD, Baerveldt G, Sunalp MA, Riekhof FT. Management of the hypotonous cyclodialysis cleft. Ophthalmology 1991;98:9:1384-1393.
6. Gentile RC, Pavlin CJ, Liebmann JM, et al. Diagnosis of traumatic cyclodialysis by ultrasound biomicroscopy. Ophthalmic Surg Lasers 1996;27:2:97-105.
7. Aminlari A, Callahan CE. Medical, laser, and surgical management of inadvertent cyclodialysis cleft with hypotony. Arch Ophthalmol (Chicago, Ill 1960) 2004;122:3:399-404.
8. Partamian LG. Treatment of a cyclodialysis cleft with argon laser photocoagulation in a patient with a shallow anterior chamber. Am J Ophthalmol 1985;99:1:5-7.
9. Brown S V, Mizen T. Transscleral diode laser therapy for traumatic cyclodialysis cleft. Ophthalmic Surg Lasers 1997;28:4:313-317.
10. Amini H, Razeghinejad MR. Transscleral diode laser therapy for cyclodialysis cleft induced hypotony. Clin Experiment Ophthalmol 2005;33:4:348-350.
11. Küchle M, Naumann GO. Direct cyclopexy for traumatic cyclodialysis with persisting hypotony. Report in 29 consecutive patients. Ophthalmology 1995;102:2:322-333.
12. Hoerauf H, Roider J, Laqua H. Treatment of traumatic cyclodialysis with vitrectomy, cryotherapy, and gas endotamponade. J Cataract Refract Surg 1999;25:9:1299-1301.
13. Agrawal P, Shah P. Long-term outcomes following the surgical repair of traumatic cyclodialysis clefts. Eye 2013;27:12:1347-1352.