Kimberly Drenser, MD, PhD, Antonio Capone Jr., MD, Royal Oak, Mich.
Retinopathy of prematurity remains a leading cause of blindness worldwide.1 ROP is characterized by avascular peripheral retina, intraocular dysregulation of vascular endothelial growth factor, and pathologic vasculogenesis. Most newborns with ROP demonstrate initial manifestations by post-menstrual age 32 weeks and reach the threshold for ablative treatment by 37 weeks.2-5 Approximately 10 percent of treated patients progress to retinal detachment despite sufficient laser treatment and subsequently require vitrectomy.6 A subset of patients with particularly aggressive disease (aggressive posterior ROP, or APROP) tend to progress early and rapidly to retinal detachment despite timely ablation.7
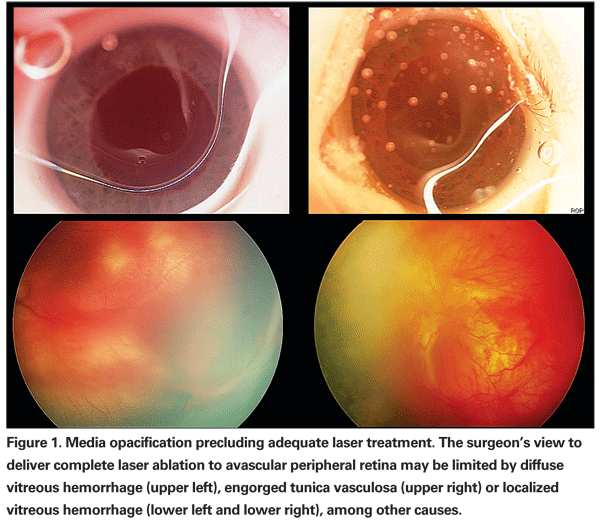
Infants who reach a treatment threshold of ROP are currently managed with laser ablation of avascular retina. Most cases of disease progression following laser ablation result from inadequate treatment or a delay in treatment after reaching threshold. Even in experienced hands, complete ablation in a single session may be difficult. Consequently, we routinely schedule a second session of laser photocoagulation in eyes with APROP one week after initial treatment in order to identify and treat any residual skip areas. In some cases, media opacity or poor pupillary dilation may limit the surgeon's view, thereby precluding complete laser ablation (See Figure 1).
The last few years have seen an increased understanding of the pathophysiology and genetics of ROP. This article discusses those advances and their implications for evaluation and treatment of ROP.
The Role of VEGF in ROP
Vascularization of the human retina extends radially from the optic disc, reaching the retinal periphery by full term.8 Vascular endothelial growth factor plays a crucial role in this process. As the neurosensory retina matures and metabolic demands increase, a relative hypoxia occurs in the avascular peripheral retina. VEGF is released by the avascular tissue, and the resulting VEGF gradient drives retinal vascularization towards the retinal periphery.9,10
VEGF dysregulation in the premature infant occurs in two stages. In the first stage, retinal vascular maturation slows as intravitreal VEGF levels decrease, while in the second stage retinal vasculature grows aberrantly as endogenous VEGF levels increase secondary to prolonged persistence of avascular retina.11 Because complete retinal vascularization does not occur until full term, premature infants are delivered into a hyperoxic ex utero environment while the peripheral retina remains incompletely vascularized.9 Hyperoxia acts to down-regulate VEGF produced by the avascular peripheral retina, and the normal process of retinal vascularization is impeded.
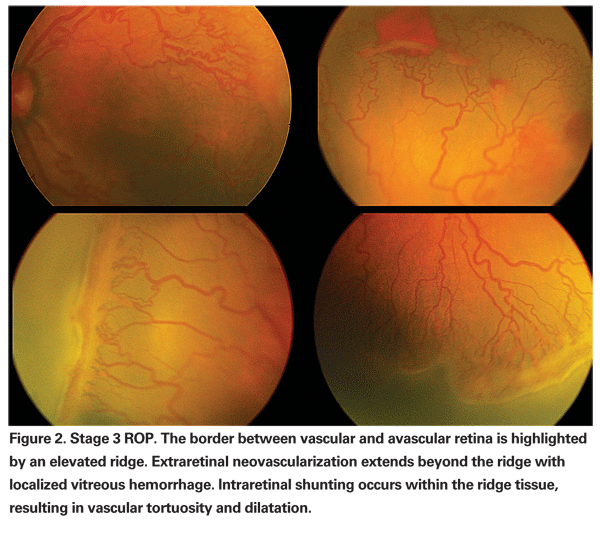
In most premature infants, retinal vascularization eventually resumes without significant sequelae. However, in some infants the prolonged tissue hypoxia experienced by an avascular retinal periphery causes an abnormal increase in VEGF production.10,12 Instead of resuming the normal maturation process, pathologic angiogenesis occurs and intra-retinal shunting and extra-retinal neovascularization develop (See Figure 2).
Anti-VEGF Therapy for ROP
Peripheral ablation is not universally effective in eliciting regression of ROP (See Figure 3). This is particularly true for APROP, which typically afflicts profoundly premature neonates.7 In this subset of infants, progression of ROP to bilateral retinal detachment and blindness may occur despite timely and complete peripheral retinal ablation.
The advent of anti-VEGF drugs has raised the possibility of treating selected cases of ROP off-label with these medications. In a study published in Ophthalmology, we took undiluted vitreous samples from the mid-vitreous of children who developed stage IV ROP despite standard-of-care laser.13 At the time of surgery the eyes were graded as vascularly active or vascularly inactive and compared to control eyes undergoing extraction of uncomplicated congenital cataract. Our findings confirmed that VEGF upregulation occurs in ROP retinal detachments and more so in eyes with persistent vascular activity. Such eyes are potential candidates for anti-VEGF medications.
As VEGF is required in the developing retina for normal angiogenesis, the goal of treatment is to quench the excessive levels of VEGF in the vitreous rather than to penetrate retinal tissue. Furthermore, as VEGF activity is endogenously down-regulated around the time of the due date, it is likely that premature infants will only require one or perhaps two injections of anti-VEGF medications to prevent progression of disease. For this reason, bevacizumab (Avastin) is a more desirable drug than ranibizumab (Lucentis) for treatment of ROP. While the two medications work through an identical mode of action, bevacizumab is a larger molecule than ranibizumab and may therefore penetrate the retina to a lesser degree.
Several small case series in the literature have documented the use of intravitreal bevacizumab in eyes with APROP and classic ROP refractory to peripheral laser ablation.14-16 Vascular activity decreased substantially in most of these patients, but progressive tractional retinal detachment occurred in a minority of eyes following bevacizumab injection.17,18
The largest experience to date of intravitreal bevacizumab for treatment of ROP is a combined series from
The next appropriate step would be a prospective clinical trial to evaluate the safety and efficacy of treatment with bevacizumab for improving structural outcomes in ROP. This is the mission of the BLOCK-ROP study, a multicenter prospective longitudinal cohort study. Phase I is an investigator-initiated multicenter Investigational New Drug study to evaluate the safety of intravitreal bevacizumab administered in a single dose as rescue therapy in one eye of infants who have not responded to standard of care laser in both eyes. Enrollment for Phase I began in June of 2008 and was anticipated to be complete by March of 2009. Assuming no untoward ocular or systemic events in Phase I, the study group will proceed to a prospective, multicenter, head-to-head comparison of intravitreal bevacizumab to standard-of-care peripheral retinal ablation.
Genetic Susceptibility
Numerous studies have delineated the role of VEGF in the pathogenesis of ROP, but no clear explanation exists for the vastly different rates of disease progression in patients with similar clinical risk factors. Studies of ROP concordance rates in twins suggest a genetic predisposition to more severe disease.21 However, studies of individual genes have not yielded consistent correlations with clinical progression of disease.
The signaling pathways potentially involved in VEGF dysregulation are complex. The Wnt pathway is perhaps the best studied of these and plays a role in vasculogenesis of the retina. Frizzled-4 acts as a receptor in the Wnt signaling pathway, and the receptor is activated by a variety of ligands including Wnt-3a, Wnt-8, Wnt-5a and Norrin.
Furthermore, multiple distinct pathways exist downstream of the activated receptor-ligand complex. Each of these components is a candidate for genetic predisposition to advanced ROP, but these components likely represent only a minority of all components involved in relevant signaling pathways.
The study of genetic predisposition to ROP has taken a shotgun approach, focusing on a few specific candidate genes or alleles rather than the entire signal pathway upstream to VEGF. This approach may explain why low serum levels of IGF-I and high vitreous levels of VEGF correlate with ROP severity, but no study of any single gene has yielded a consistent causal relationship.13,22-29 Several studies have identified Norrin mutations in patients with advanced ROP.27,30-33 Similarly, mutations in Frizzled-4 have been identified in a minority of patients with advanced ROP. (Drenser KA, unpublished data).
Studies of alleles for VEGF and IGF-1 have occasionally identified alleles correlated with ROP development or severity, but allelic variation has poor predictive value and some studies with positive findings directly contradict each other.23,24,29,34
If VEGF is the common downstream dysregulated factor in patients with advanced ROP, many upstream genetic alterations could potentially start the cascade of dysregulation.
Focusing on any single gene to the exclusion of others is short-sighted, and the pathways involved in VEGF regulation are sufficiently complex that logically identifying candidate genes may be inadequate.
Effective genetic evaluation for predisposition to ROP will require identification of a sufficiently wide range of mutations as well as the development of a simple and inexpensive genetic analysis technique that can be extended to facilities without capital-intensive laboratory equipment.
Telemedicine ROP Evaluation
Without the ability to successfully predict which newborns are at risk for ROP, beyond birth weight and gestational age criteria, large numbers of infants must be evaluated in order to identify the small proportion requiring close monitoring and treatment. In the United States both manpower and medico-legal concerns limit the pool of ophthalmologists available and willing to perform ROP evaluation.35 Telemedicine is a tool that may help alleviate this problem.
Telemedicine ROP evaluation does not replace bedside binocular indirect ophthalmoscopy examination entirely. Instead, photographic evaluation functions to identify a minority of eyes with high-risk characteristics for which BIO and possibly treatment are warranted. While a subspecialty trained ophthalmologist must still be available within reach of the local health care system, the resource burden imposed upon this physician is dramatically reduced compared to bedside evaluation of all at-risk infants.
A successful telemedicine ROP evaluation program has several requirements. Images must be obtained by trained personnel and transmitted to a remote reading center rapidly and securely. Because intervention may be required within 36 to 48 hours, images must be read and interpretations transmitted back to the consulting hospital within a short time frame. In addition, image interpretation must be standardized with quality controls.
For this reason, the
An effective screening program errs on the side of false-positive identification rather than false negatives, since there is little downside to an additional bedside examination, while delayed identification of severe ROP may have dire consequences. Several substantial studies published last year demonstrated high sensitivity and high negative predictive value, confirming that telemedicine ROP evaluation is reaching the point of maturity required for widespread implementation.
The SUNDROP network based at Stanford University provides photographic screening to local neonatal intensive care units in lieu of bedside examination.35,36 The network's published 18-month results noted sensitivity of 100 percent and specificity of 98.9 percent in 194 eyes of 97 infants receiving 443 examinations.36 The negative predictive value was 100 percent with a primary outcome of treatment-warranting disease.
The largest telemedicine ROP study to compare photographs with BIO was the Photo-ROP study, a multicenter study comparing weekly standardized photographs to BIO by an experienced specialist. A total of 51 patients received 300 examinations, and primary outcomes included both referral-warranting ROP (defined as clinically significant ROP, or CSROP) and treatment-warranting ROP based on ETROP criteria.37 Using bedside examination as a gold standard, photographic screening demonstrated sensitivity of 92 percent for both CSROP and ETROP criteria, and negative predictive values were 76 percent for CSROP and 97 percent for ETROP.
In another large series using bedside examination as a gold standard, 72 eyes of 36 infants received 371 examinations at weekly to biweekly intervals based on standard Canadian ROP evaluation guidelines.38 Using a primary outcome of referral-warranting ROP, photographic evaluation demonstrated sensitivity of 100 percent and specificity of 96 percent. The negative predictive value was 100 percent. Of note, referral-warranting ROP was detected a week earlier by photography than by bedside examination in the 10 patients who progressed and required treatment.
Telemedicine ROP evaluation has already taken hold in various centers in the
Dr. Ranchod is a fellow in vitreoretinal diseases and surgery at Associated Retinal Consultants in Royal Oak, Mich. Dr. Drenser is the director of ophthalmic research for the Department of Ophthalmology at William Beaumont Hospital in Royal Oak, Michigan and a clinical assistant professor of biomedical sciences at Oakland University in Auburn Hills, Mich. Dr. Capone is the director of the fellowship in vitreoretinal diseases and surgery at Associated Retinal Consultants, director of the Vision Research Foundation at ARC, and a clinical associate professor of biomedical sciences at Oakland University.
Contact Dr. Ranchod at Associated Retinal Consultants,
1. Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP). Arch Ophthalmol 2006;124(1):24-30.
2. Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial I: Primary outcomes. Pediatrics 2000;105(2):295-310.
3. Palmer EA, Flynn JT, Hardy RJ, et al. Incidence and early course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1991;98:1628-40.
4. Schaffer DB, Palmer EA, Plotsky DF, et al. Prognostic factors in the natural course of retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Ophthalmology 1993;100:230-7.
5. Reynolds JD, Dobson V, Quinn GE, et al. Evidence-based screening criteria for retinopathy of prematurity: Natural history data from the CRYO-ROP and LIGHT-ROP studies. Arch Ophthalmol 2002;120:1470-6.
6. Good WV. Final results of the Early Treatment for Retinopathy of Prematurity (ETROP) randomized trial. Trans Am Ophthalmol Soc 2004;102:233-48; discussion 48-50.
7. The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005;123:991-9.
8. Roth AM. Retinal vascular development in premature infants. Am J Ophthalmol 1977;84:636-40.
9. Lutty GA, Chan-Ling T, Phelps DL, et al. Proceedings of the Third International Symposium on Retinopathy of Prematurity: An update on ROP from the lab to the nursery (November 2003, Anaheim, Calif.). Mol Vis 2006;12:532-80.
10. Pierce EA, Avery RL, Foley ED, et al. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci
11. Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10(2):133-40.
12. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994;331(22):1480-7.
13. Sonmez K, Drenser KA, Capone A, Jr., Trese MT. Vitreous levels of stromal cell-derived factor 1 and vascular endothelial growth factor in patients with retinopathy of prematurity. Ophthalmology 2008;115(6):1065-70 e1.
14. Kusaka S, Shima C, Wada K, et al. Efficacy of intravitreal injection of bevacizumab for severe retinopathy of prematurity: a pilot study. Br J Ophthalmol 2008;92(11):1450-5.
15. Nazari H, Parvaresh MM, Hashemi M, et al. Intravitreal bevacizumab for the treatment of Aggressive Posterior Retinopathy of Prematurity unresponsive to conventional laser therapy. Retina 2009;In press.
16. Mintz-Hittner HA, Kuffel RR, Jr. Intravitreal injection of bevacizumab (avastin) for treatment of stage 3 retinopathy of prematurity in zone I or posterior zone II. Retina 2008;28(6):831-8.
17. Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefe's Arch Clin Exp Ophthalmol 2008;246(7):1061-3.
18. Lalwani GA, Berrocal AM, Murray TG, et al. Off-label use of intravitreal bevacizumab (Avastin) for salvage treatment in progressive threshold retinopathy of prematurity. Retina 2008;28:S13-S8.
19. Martinez-Castellanos MA, Domenzain-Afendulis JL, Hernandez-Rojas ML, et al. Safety and efficacy of intravitreal bevacizumab for retinopathy of prematurity. Invest Ophthalmol
20. Quiroz-Mercado H, Martinez-Castellanos MA, Hernandez-Rojas ML, et al. Antiangiogenic therapy with intravitreal bevacizumab for retinopathy of prematurity. Retina 2008;28:S19-S25.
21. Bizzarro MJ, Hussain N, Jonsson B, et al. Genetic susceptibility to retinopathy of prematurity. Pediatrics 2006;118(5):1858-63.
22. Balogh A, Derzbach L, Vannay A, Vasarhelyi B. Lack of association between insulin-like growth factor I receptor G(+3174)A polymorphism and retinopathy of prematurity. Graefe's Arch Clin Exp Ophthalmol 2006;244(8):1035-8.
23. Cooke RW, Drury JA, Mountford R, Clark D. Genetic polymorphisms and retinopathy of prematurity. Invest Ophthalmol Vis Sci 2004;45:1712-5.
24. Dunai G, Vasarhelyi B, Szabo M, et al. Published genetic variants in retinopathy of prematurity: Random forest analysis suggests a negligible contribution to risk and severity. Curr Eye Res 2008;33(5):501-5.
25. Haider MZ, Devarajan LV, Al-Essa M, et al. Missense mutations in norrie disease gene are not associated with advanced stages of retinopathy of prematurity in Kuwaiti arabs. Biol Neonate 2000;77(2):88-91.
26. Hellstrom A, Perruzzi C, Ju M, et al. Low IGF-I suppresses VEGF-survival signaling in retinal endothelial cells: direct correlation with clinical retinopathy of prematurity. Proc Natl Acad Sci USA 2001;98(10):5804-8.
27. Hutcheson KA, Paluru PC, Bernstein SL, et al. Norrie disease gene sequence variants in an ethnically diverse population with retinopathy of prematurity. Mol Vis 2005;11:501-8.
28. Kim JH, Yu YS, Kim J, Park SS. Mutations of the Norrie gene in Korean ROP infants. Korean J Ophthalmol 2002;16(2):93-6.
29. Vannay A, Dunai G, Banyasz I, et al. Association of genetic polymorphisms of vascular endothelial growth factor and risk for proliferative retinopathy of prematurity. Pediatr Res 2005;57(3):396-8.
30. Dickinson JL, Sale MM, Passmore A, et al. Mutations in the NDP gene: Contribution to Norrie disease, familial exudative vitreoretinopathy and retinopathy of prematurity. Clin Experiment Ophthalmol 2006;34(7):682-8.
31. Hiraoka M, Berinstein DM,
32. Shastry BS,
33. Wu WC, Drenser K, Trese M, et al. Retinal phenotype-genotype correlation of pediatric patients expressing mutations in the Norrie disease gene. Arch Ophthalmol 2007;125:225-30.
34. Holmstrom G, van Wijngaarden P, Coster DJ, Williams KA. Genetic susceptibility to retinopathy of prematurity: the evidence from clinical and experimental animal studies. Br J Ophthalmol 2007;91(12):1704-8.
35.
36. Silva RA, Murakami Y, Jain A, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): 18-month experience with telemedicine screening. Graefe's Arch Clin Exp Ophthalmol 2009;247(1):129-36.
37. Capone A, Jr., Ells
38. Ells AL, Holmes JM, Astle WF, et al. Telemedicine approach to screening for severe retinopathy of prematurity: A pilot study. Ophthalmology 2003;110:2113-7.



