Pegaptanib, a selective inhibitor of the VEGF165 isoform, was the first anti-VEGF agent approved for an ocular indication in 2004,1 followed by non-selective VEGF inhibitors with a more robust efficacy profile: ranibizumab in 20062 and aflibercept in 2011.3 Off-label intravitreal bevacizumab, also a non-selective VEGF inhibitor, has been in robust use since 20054 and retains predominant market share due to having similar efficacy to ranibizumab combined with a distinct pricing advantage.
The popularity of non-selective anti-VEGF agents has largely been driven by their apparent efficacy that is measurable both functionally and anatomically. Patients, too, appreciate the merits of these anti-VEGF agents for various indications, and they do so despite knowing the burden of needing intravitreal injections every one to three months in order to realize these anatomic and functional benefits over the long term.
A lot has been said about the ocular and systemic safety of anti-VEGF agents over the last 10 years. From the beginning of the anti-VEGF era in ophthalmology in 2004, retina specialists and clinical trialists were appropriately concerned about the potential ocular and systemic side effects of constitutively inhibiting VEGF, albeit in minute anti-VEGF doses delivered into the relatively isolated intraocular environment.
|
Our views on the systemic safety profile of these agents have likely been significantly prejudiced by the mechanism of action of these agents vis-à-vis what is known about the systemic side effects of similar drugs delivered intravenously in manifold higher doses for systemic management of disease.6 These concerns have perhaps been mitigated by empiric data from clinical trials for ocular indications as well as from our own clinical experience with these agents. As we will see, however, subgroup analyses of the existing data can lead to persistently unanswered questions regarding the relative systemic safety of each of these agents. Additional concerns have been raised by recent pharmacokinetic studies for each of these agents.
Ocular Safety
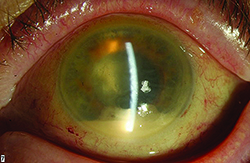
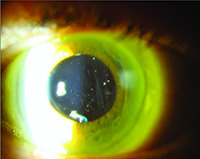
It is clear from our clinical trial and everyday clinical experience that the aging eye tolerates multiple intravitreal injections of anti-VEGF agents very well. The most concerning risk is the development of endophthalmitis, which multiple large retrospective studies have demonstrated occurs roughly once out of every 5,000 injections (endophthalmitis rate equal to 0.02%).7 Not all post-injection cases of endophthalmitis due to a bacterial source are irreversibly blinding, however, there is a relatively high proportion of culture-positive cases that have the virulent Streptococcal species as the causative organism. Many of these Streptococcal cases have dismal functional results, with visual acuity often dropping to hand motions or worse level and many cases leading to evisceration or enucleation.
While the exact source of the infectious nidus remains unknown, some evidence suggests it may be due to respiratory flora from the patient, the medical assistant or the injecting physician.8,9 Therefore, most practitioners have a no-talking policy during the injection process. While some have advocated the use of face masks, this is not the prevailing standard of care.10
Severe eye pain, with or without decreased vision, in the first two days following the antecedent intravitreal anti-VEGF is the most common symptom.7 Hypopyon, anterior chamber fibrin, cell and flare, along with vitreitis are the most common ocular signs. Because patients can have variably significant ocular irritation for the first 48 hours after a routine injection due to the povidone iodine 5% sterilizing solution applied to the ocular surface, it is important to emphasize that this pain tends to lessen over time while severe and deep eye pain that worsens over time is the most concerning sign for infectious cause of their symptoms. While the “per-injection” risk of endophthalmitis is quite low, clinical trial data has revealed the “per-patient” risk of endophthalmitis is closer to 1 percent.3
Initially it was felt to be important to provide pre- or post-injection antibiotic prophylaxis to reduce the risk of endophthalmitis.5 We later learned that this practice not only did nothing to reduce the risk of infection, it also created more antibiotic-resistant bacteria for those cases that did develop despite the use of antibiotics.11,12 As a result, the use of pre- or post-injection prophylactic antibiotics is not advocated.
Insofar as off-label intravitreal bevacizumab is only available through compounding pharmacies, there is a potential risk for contamination of bevacizumab during the aliquoting process, during transportation from the pharmacy to the physician’s office or during storage of the drug.13 It is important for physicians to become aware of their supplying pharmacy’s procedures for the above and check in with them regularly to ensure appropriate compliance. Unfortunately, outbreaks of blinding cases of endoph-thalmitis have occurred when deviation from established protocols has led to widespread contamination of bevacizumab lots.14
While retinal tears, retinal detachment, vitreous hemorrhage and traumatic iatrogenic cataract are reported risks following retinal intravitreal anti-VEGF agents, these are not only quite uncommon,5 but are also less permanently visually debilitating in general than endophthalmitis is.
|
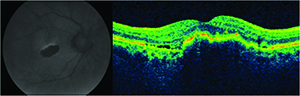
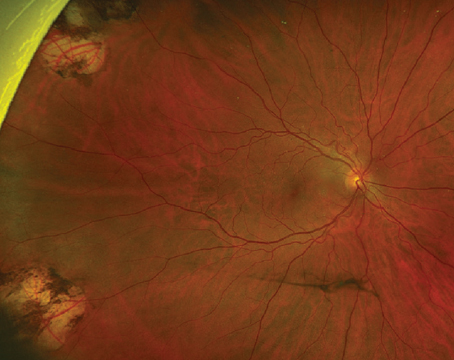
Geographic atrophy is a well-known consequence of AMD progression, but was also observed as the number one cause of decreased vision in patients undergoing ranibizumab therapy in the MARINA trial.16
It is well-known that ocular hypertension can occur transiently immediately following the bolus injection of 50 to 100 microliters of an anti-VEGF drug. What is more concerning is the potential for significant and sustained elevations in intraocular pressure elevation. This appears to occur in 3.5 percent to 11 percent of patients receiving chronic anti-VEGF agents.17-19 It is unclear whether the etiology of this is mediated by the drugs’ mechanisms of action on the trabecular meshwork, hydrostatic damage to the trabecular meshwork, outflow impairment, a combination of these, or due to some other unidentified mechanism. The key point here is to recognize the possibility of the risk (especially in patients with pre-existing risk factors for glaucoma), to monitor the IOP and optic nerve and to make the appropriate adjustments to the injection protocol when delivering subsequent anti-VEGF injections (e.g., lowering the volume of drug injection, using a larger-bore needle or increasing the injection interval).
Systemic Safety
VEGF is a potent promoter of vascular hyperpermeability. Anti-VEGF agents significantly reduce vascular hyperpermeability, and when delivered systemically, can also raise systemic arterial blood pressure.20,21 Systemic arterial hypertension is the single greatest risk factor the development of cerebrovascular accidents. Systemically delivered anti-VEGF agents are also widely known to promote the development of other thromboembolic events.20,21 Arterial thrombotic events, or ATEs, are defined as nonfatal stroke, nonfatal myocardial infarction or vascular death (including deaths of unknown cause). However, these systemic events have been observed following systemic administration of anti-VEGF agents in much larger doses than what are being injected intravitreally. Do these same risks occur with such small doses that we inject into the eye routinely?
The short answer is that we do not definitively know yet. There have been no statistically significant signals derived from Level I evidence that directly point to a greater risk for the development of ATEs for patients in the anti-VEGF cohorts compared with those in the control groups in these studies. It must be understood, however, that these rigorous clinical trials were not statistically powered to answer this specific question. Additionally, it is unlikely that clinical trials designed to answer this question will be carried out, insofar as each arm of such a study would have to include more than 10,000 patients in order to be sufficiently powered to detect a meaningful difference for these rare events. That said, there is compelling clinical trial subgroup analysis and pharmacokinetic data pointing to the possibility of a risk of ATEs from intravitreal anti-VEGF drugs.
The CATT trial, for example, revealed that there was a greater risk for serious adverse events associated with bevacizumab compared with ranibizumab even though there was no significant difference between the two drugs with respect to the risk for ATEs.22 Similarly, the IVAN study demonstrated that circulating serum VEGF levels were more significantly diminished in the bevacizumab group as compared with the ranibizumab group.23
Ranibizumab was approved for the treatment of diabetic macular edema after regulators evaluated data from the ranibizumab 0.5-mg and 0.3-mg cohorts as compared with a control group. Although each ranibizumab group had very similar efficacy data, there was an indication that the 0.5-mg group may have had a more concerning safety profile. In the pooled analysis of the RISE and RIDE ranibizumab for DME studies at three years, the ATE rate was 10.4 percent (26 of 249) with 0.5 mg ranibizumab and 10.8 percent (27 of 250) with 0.3 mg ranibizumab; the stroke rate was 4.8 percent (12 of 249) with 0.5 mg ranibizumab and 2.0 percent (five of 250) with 0.3 mg ranibizumab.24 Additionally, over three years, fatalities occurred in 6.4 pecent (16 of 249) of patients treated with 0.5 mg ranibizumab and in 4.4 percent (11 of 250) of patients treated with 0.3 mg ranibizumab.24 Although not statistically significant, these numerical differences were enough of a dose-dependent concern for both the sponsor and the regulator to agree to a 0.3-mg approved indication instead of the 0.5-mg dose that was previously approved for AMD and retinal vein occlusion.
The European Medicines Agency recently provided a Public Assessment Report (EPAR) on the use of aflibercept for neovascular AMD.25 In its analysis of the available pooled data, the agency observed that the number of cerebrovascular accidents was higher in the aflibercept group (n=35, 1.9 percent) compared with the ranibizumab group (n=2, 0.3 percent) and that the magnitude of this difference was especially noticeable when analyzing the oldest pool of patients. For those 85 years of age and older, the number of cerebrovascular accidents after one year of therapy was 20 (7.1 percent) in the aflibercept arm versus just one (1.1 percent) in the ranibizumab arm. After two years of therapy, the difference was 27 cases (9.5 percent) in the aflibercept arm compared with three cases (3.4 percent) in the ranibizumab arm.25
|
Barry Kuppermann, MD, nicely characterized the anti-VEGF patient populations at the highest risk for ATEs in an August 2014 interview. He enumerated four high-risk groups based upon available clinical trial data, clinical experience and theoretical factors. They are: diabetics; patients 85 years and older; patients with prior strokes; and premature infants. With respect to retinopathy of prematurity, although we have limited Level 1 data, there is considerable theoretical justification for concern for the use of anti-VEGF drugs in this very vulnerable patient population.29,30
Anti-VEGF agents for retinal disease are ubiquitous and very well-tolerated from an ocular perspective. While irreversible loss of vision following intravitreal anti-VEGF agents due to endophthalmitis is rare, it is a continuing risk that requires constant vigilance and attention to detail by the treating physician.
The superior efficacy of these agents over those treatments previously available is readily apparent, but clearly they remain suboptimal treatments. They are lacking not just in the fact that such frequent administration is required to achieve visual acuity and anatomic improvements, but also by their very mechanism of action that makes them so efficacious. Inhibiting systemic VEGF at such low levels opens the door to the potential for the development of ATEs. While clinical trial data have not definitively demonstrated that this risk is real, the data we do have is sufficiently concerning to warrant continued investigation and monitoring of this potential problem to determine if there are drug-specific safety characteristics that would favor one over the other. REVIEW
Dr. Moshfeghi is an associate physician and surgeon at Retina Associates of Kentucky, in Lexington.
1. Gragoudas ES, Adamis AP, Cunningham ET Jr, Feinsod M, Guyer DR; VEGF Inhibition Study in Ocular Neovascularization Clinical Trial Group. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 2004;351(27):2805-16.
2. Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355(14):1419-31.
3. Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD, Kirchhof B, Ho A, Ogura Y, Yancopoulos GD, Stahl N, Vitti R, Berliner AJ, Soo Y, Anderesi M, Groetzbach G, Sommerauer B, Sandbrink R, Simader C, Schmidt-Erfurth U; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537-48.
4. Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology 2006;113:363-372.
5. Jager RD, Aiello LP, Patel SC, Cunningham ET Jr. Risks of intravitreous injection: A comprehensive review. Retina 2004;24:676-98.
6. Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, Venkatraman AS.Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: Twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology 2006;113:2002.
7. Moshfeghi AA, Rosenfeld PJ, Flynn HW Jr, Schwartz SG, Davis JL, Murray TG, Smiddy WE, Berrocal AM, Dubovy SR, Lee WH, Albini TA, Lalwani GA, Kovach JL, Puliafito CA. Endophthalmitis after intravitreal vascular [corrected] endothelial growth factor antagonists: A six-year experience at a university referral center. Retina 2011;31:662-668.
8. Wen JC, McCannel CA, Mochon AB, Garner OB. Bacterial dispersal associated with speech in the setting of intravitreous injections. Arch Ophthalmol 2011;129:1551-4.
9. Doshi RR, Leng T, Fung AE. Reducing oral flora contamination of intravitreal injections with face mask or silence. Retina 2012;32:473-6.
10. Schimel AM, Scott IU, Flynn HW Jr. Endophthalmitis after intravitreal injections: should the use of face masks be the standard of care? Arch Ophthalmol 2011;129:1607-9.
11. Kim SJ, Toma HS. Antimicrobial resistance and ophthalmic antibiotics: 1-year results of a longitudinal controlled study of patients undergoing intravitreal injections. Arch Ophthalmol 2011;129:1180-8.
12. Kim SJ, Toma HS. Ophthalmic antibiotics and antimicrobial resistance: A randomized, controlled study of patients undergoing intravitreal injections. Ophthalmology 2011;118:1358-63.
13. Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP. Avastin doesn’t blind people, people blind people. Am J Ophthalmol 2012;153:196-203.
14. Goldberg RA, Flynn HW Jr, Miller D, Gonzalez S, Isom RF. Streptococcus endophthalmitis outbreak after intravitreal injection of bevacizumab: One-year outcomes and investigative results. Ophthalmology 2013;120:1448-53.
15. Cunningham ET Jr, Feiner L, Chung C, Tuomi L, Ehrlich JS. Incidence of retinal pigment epithelial tears after intravitreal ranibizumab injection for neovascular age-related macular degeneration. Ophthalmology 2011;118:2447-52.
16. Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B; MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011;118:523-30.
17. Freund KB. Can anti-VEGF therapy cause glauoma? Available at: http://www.retinacme.com/new-developments-in-retinal-pharmacotherapy/2013/06_june/can-anti-vegf-therapy-cause-glaucoma/cme-information, accessed September 8, 2014.
18. Bakri SJ, Moshfeghi DM, Francom S, Rundle AC, Reshef DS, Lee PP, Schaeffer C, Rubio RG, Lai P. Intraocular pressure in eyes receiving monthly ranibizumab in 2 pivotal age-related macular degeneration clinical trials. Ophthalmology 2014;121:1102-8.
19. Pershing S, Bakri SJ, Moshfeghi DM. Ocular hypertension and intraocular pressure asymmetry after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmic Surg Lasers Imaging Retina 2013 Sep-Oct;44(5):460-4.
20. Bevacizumab full prescribing information. Available at: http://www.gene.com/download/pdf/avastin_prescribing.pdf, accessed September 8, 2014.
21. Z-aflibercept full prescribing information. Available at: https://www.regeneron.com/zaltrap/zaltrap-fpi.pdf, accessed September 8, 2014.
22. CATT Research Group. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–908.
23. Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, Reeves BC. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: One-year findings from the IVAN randomized trial. The IVAN Study Investigators. Ophthalmology 2012;119:1399-411.
24. Brown DM, Nguyen QD, Marcus DM, et al; RIDE and RISE Research Group. Long-term outcomes of ranibizumab therapy for diabetic macular edema: The 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120:2013-2022.
25. European Medicines Agency. European Public Assessment Report (EPAR). Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002392/WC500135744.pdf, accessed September 8, 2014.
26. Ranibizumab full prescribing information. Available at: http://www.gene.com/download/pdf/lucentis_prescribing.pdf, accessed September 8, 2014.
27. Aflibercept full prescribing information. Available at: http://www.regeneron.com/Eylea/eylea-fpi.pdf, accessed September 8, 2014.
28. Avery RL, Castellarin AA, Steinle NC, et al. Systemic pharmacokinetics following intravitreal injections of ranibizumab, bevacizumab or aflibercept in patients with neovascular AMD. Br J Ophthalmol 2014;0:1-6.
29. Mintz-Hittner HA, Kennedy KA, Chuang AZ; BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med 2011;364:603-15.
30. Moshfeghi DM, Berrocal AM. Retinopathy of prematurity in the time of bevacizumab: Incorporating the BEAT-ROP results into clinical practice. Ophthalmology 2011;118:1227-8.