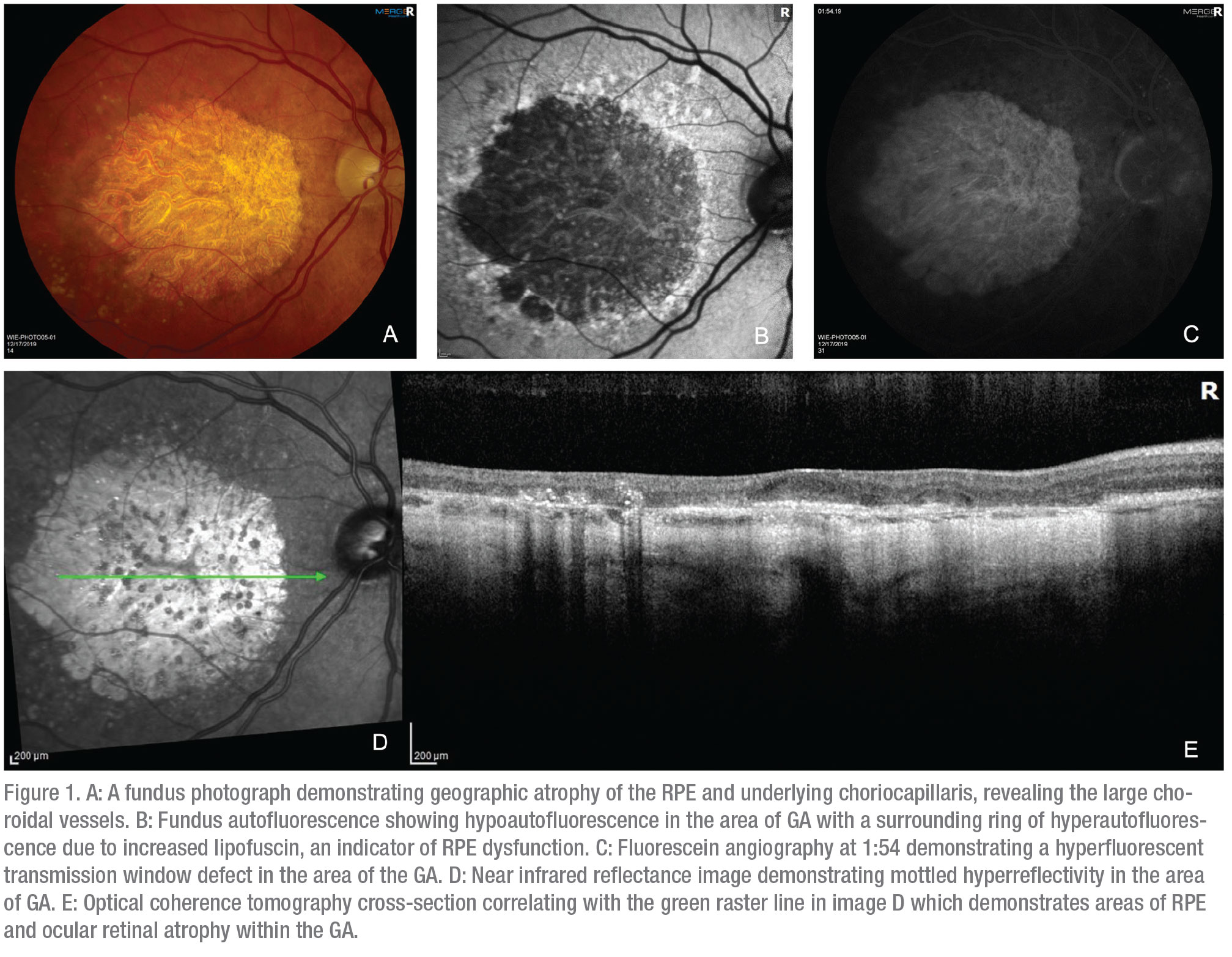
Age-related macular degeneration is a leading cause of blindness in patients 65 years and older.1 Geographic atrophy (GA) is an advanced form of age-related macular degeneration characterized by loss of the retinal pigment epithelium and photoreceptors in the macula (Figure 1).2 AMD is a multifactorial disease influenced by both environmental factors such as smoking and genetic factors involving the complement system.3 For example, Age-Related Maculopathy Susceptibility 2 (ARMS2) and Complement Factor H (CFH) are two of many genes that have been identified and associated with an increased risk of AMD.
However, despite our growing understanding of the genetics and pathophysiology of dry AMD, an effective treatment remains elusive. In contrast, the advent of anti-vascular endothelial growth factor therapy for wet AMD has enabled us to not only preserve, but in many cases, improve vision. Moreover, there are several potential treatments in the pipeline for wet AMD that may provide enhanced durability, such as the ranibizumab port delivery system, abicipar, faricimab and gene therapy. Unfortunately, there has yet to be a similar breakthrough for GA. Due to its complexity, many therapeutic approaches have been considered, including visual cycle modulation, neuroprotection, cell-based therapy, inflammation suppression and complement inhibition. In this review, we examine the past and current research into a pharmaceutical treatment for atrophic AMD.
Visual Cycle Modulation
Visual cycle modulators are oral medications that target enzymes in the visual cycle. In phototransduction, photoreceptors exert a high metabolic demand, which results in increased production of metabolic waste products. Accumulation of these byproducts may lead to increased inflammation, which is implicated in the development of GA. Modulating the visual cycle may mitigate this process and reduce inflammation and GA. A drug that can be delivered orally is especially attractive; however, a consequence of modulating the visual cycle is that dark adaptation and low-light vision are often adversely affected.
Following are the two highest-profile visual cycle modulators that have been studied.
Emixustat hydrochloride (ACU-4429) is an oral non-retinoid small molecule that inhibits the visual cycle enzyme isomerohydrolase, RPE65.4 It was studied in the Safety and Efficacy Assessment Treatment Trials of Emixustat hydrochloride (SEATTLE) study, a large randomized clinical trial for GA by Bascom Palmer’s Philip Rosenfeld, MD, and co-workers. In the study, 508 people were enrolled with GA secondary to macular degeneration. Unfortunately, the Phase IIb/III clinical trial showed that oral emixustat hydrochloride was ineffective at reducing the progression of GA.5
ALK-001 is a modified form of vitamin A that replaces natural vitamin A in the body. The modified vitamin A forms toxic vitamin A dimers more slowly, which is postulated to slow the accumulation of toxic end products and therefore slow the development and/or progression of AMD.6 However, this effect may also impact the visual cycle by depleting vitamin A and causing delayed dark adaptation. It’s currently being studied in a Phase III clinical trial for GA.
Neuroprotection
Neuroprotection has been investigated as a possible solution for the problem of progressive cellular damage and eventual cell loss that occurs in atrophic AMD. Pharmacologic agents with cyto- and neuroprotective properties may help protect at-risk neuroretinal tissue by increasing its resilience and resistance to cellular injury, thereby providing a defense against GA progression. So far, brimonidine tartrate (Allergan) is the only agent that has shown possible neuroprotective properties that might be beneficial in GA.
Brimonidine is an alpha-2 adrenergic agonist that’s an established topical ophthalmic intraocular pressure-lowering agent. However, studies in animal models with systemic administration have demonstrated that it also has neuroprotective properties, though clinical trials have yet to confirm similar efficacy in humans.7,8 A Phase IIa clinical trial investigated intravitreal brimonidine for GA, administered by a delayed-delivery system (DDS) implant. The DDS is a biodegradable polymer drug delivery system similar to the dexamethasone implant Ozurdex (Allergan) with a 22-gauge needle attached to a proprietary applicator system. It’s designed to release brimonidine over six months; patients receive a second injection at month six. The study evaluated 113 patients over a two-year period; the primary endpoint was the change in size of GA lesion area from baseline to month 12. Patients were randomized to study-eye treatment with brimonidine tartrate DDS 200 µg (n=49), 400 µg (n=41) or sham procedure (n=23). Compared with the control group, the rate of GA progression was lower in the brimonidine groups, but the difference wasn’t statistically significant. A Phase IIb trial (BEACON) using the higher dose of brimonidine in a new formulation showed a reduction in GA progression, and two Phase III trials are being planned (IMAGINE and ENVISION).9
Cell-based Therapies
The immune-privileged environment of the subretinal space makes it a unique target for cell-based therapy, which consists of two approaches: stem cell- and non-stem cell-based therapies. The non-stem cell approach is centered on delivering cells which can produce protective factors that are deficient within the extracellular milieu. The stem cell approach involves delivering new retinal pigment epithelium cells to help maintain the health of the remaining retinal photoreceptors, which may allow damaged or dormant light-sensitive cells to return to function. Following is a look at several of the more promising cell-based therapies in development.
Janssen Pharmaceuticals evaluated a non-stem cell-based therapy with palucorcel (CNTO-2476), which uses human umbilical cord tissue-derived cells (hUTC).10 The initial study was a Phase I/IIa, multicenter study of subretinal palucorcel in 35 patients with bilateral GA and exudative neovascular AMD, who had no other ophthalmic conditions, and were suitable candidates for ophthalmic surgery. In the first phase of the study, 29 participants received a single, subretinal dose of palucorcel of 27 µl (3.03 X 105 viable cells), 50 µl (6.03 X 104, 1.23 X 105 viable cells) or 50 µl (5.63 X 105 viable cells) via an external approach, superior to the GA lesion. The Phase IIa study was eventually suspended due to the significant risk of adverse events.
Although palucorcel was well-tolerated in the Phase I/IIa study, the ab externo surgical approach required to access the subretinal space with a microcatheter delivery system was associated with a high rate of retinal perforations (13/35 operated subjects) and retinal detachments (6/35 operated subjects).11 Only 15 percent of the patients had adverse events related to palucorcel alone, and those may have been exacerbated by the surgery and delivery as well, according to the study.12 Although patients had significant adverse events, the mean BCVA gain at one year was more than four letters, while a quarter of the patients gained three lines of vision or more. The average vision loss at one year was two letters for untreated fellow eyes.
In contrast, Ocata Therapeutics (recently acquired by Astellas Pharma) assessed a stem cell-based approach using human embryonic stem cells (hESC). The Phase I/II safety studies of hESC MA09-hRPE assessed an initial group of nine patients with AMD and nine patients with Stargardt’s Disease.13 Three dose cohorts (50,000, 100,000 and 150,000 cells) were assessed. There was no evidence of adverse proliferation, rejection or serious ocular or systemic safety issues related to the transplanted tissue. Adverse events were associated with vitreoretinal surgery and immunosuppression, including one case of endophthalmitis. After transplantation, 72 percent of patients had increased subretinal pigment at the atrophic area border and, at one year, visual function was improved in nine eyes and stable in seven. Optical coherence tomography showed reconstitution or thickening of the RPE layer in some subjects.14 The related, Phase II PORTRAY trial of hESC-derived RPE cells in dry AMD was aimed at assessing graft rejection strategies and secondarily at change in area of GA and in visual acuity. The trial was initially suspended prior to enrollment due to changes in the study design and cell line but is now enrolling.
Another stem cell-based approach involving a subretinal implant is being investigated by a group led by USC’s Keck Eye Institute researchers Amir Kashani, MD, and Mark Humayun, MD, in a trial sponsored by the implant’s maker, Regenerative Patch Technologies.15 The composite implant, termed the California Project to Cure Blindness-Retinal Pigment Epithelium 1 (CPCB-RPE1), is a polarized monolayer of human embryonic stem cell-derived RPE (hESC-RPE) on an ultrathin, synthetic parylene substrate designed to mimic Bruch’s membrane. A total of 16 patients with advanced dry AMD were enrolled in the Phase I/IIa study. Intraoperative OCT-based measurements performed before and after implant placement showed that a significant area of GA could be targeted and covered with the implant. In at least three subjects, the implant covered 100 percent of the area of GA, and in the last 14 patients, the area of GA coverage was greater than half. None of the implanted eyes showed progression of vision loss and one eye improved by 17 letters.16 The concurrent structural and functional findings suggest that CPCB-RPE1 may improve visual function.15,17
Another group is working on a similar RPE patch as a part of The London Project to Cure Blindness.18,19
 |
Anti-inflammatory Agents
Tetracyclines are broad-spectrum antibiotic compounds that also exhibit non-antimicrobial anti-inflammatory properties. They have been shown to reduce reactive oxygen species, inhibit matrix metalloproteinases involved in the breakdown of the barrier between the RPE and Bruch’s membrane, inhibit caspase activation, prevent complement activation and inhibit cytokine production through their effects on microglia and T-cell activation.20,21
One example of an anti-inflammatory used in this capacity is doxycyline (Oracea, Galderma Laboratories, Fort Worth, Texas), which is being investigated in a Phase III clinical trial (TOGA) for the treatment of GA. In the study, 286 patients have been enrolled, and they’ll complete a six-month observation phase followed by a 24-month treatment phase. Randomization occurs at month six in a 1:1 ratio, to either 40 mg of oral Oracea or placebo capsule to be taken once-daily for 24 months. The results are pending.22
Another treatment is the use of FHTR2163 (Genentech/Roche), a new antibody delivered by intravitreal injection that inhibits HTRA1, a serine protease gene associated with GA.23 Recent studies have also identified HTRA1 as a major risk factor for wet AMD.23 A Phase II clinical trial, GALLEGO, is under way to further investigate this drug. A total of 285 participants will be enrolled.24
Complement Inhibition
Overactivity of the complement system appears to play a pivotal role in the pathogenesis of GA.25,26,27 The complement system, a critical component of the innate immune system, is composed of three biochemical pathways: classical; lectin; and alternative. The classical pathway is largely driven by the formation of antibody-antigen complexes, while the lectin pathway is activated by polysaccharides on microbial surfaces. In contrast, the alternative pathway is activated by surface pathogens, but doesn’t rely on immune complex formation.25,28 All three pathways converge on the cleavage of C3 (the most abundant complement protein in the blood) into activation products C3a, C3b, C5a and formation of the membrane attack complex (MAC=C5b-9) and ultimately cell death.28 Complement factor H (CFH) and complement factor I (CFI) are negative regulators of the alternative pathway that work to inactivate C3b and interrupt the pro-inflammatory response.
C5 inhibitors such as eculizumab (Soliris; Alexon, Boston) were designed to inhibit the formation of C5a and the membrane attack complex (MAC=C5b-9). Eculizumab, a humanized monoclonal antibody derived from the murine anti-human C5 antibody, was evaluated in the Phase II COMPLETE Study (NCT00935883).29 The COMPLETE study enrolled 30 patients age 50 years and older with GA and visual acuity of 20/63 or better (ETDRS).Despite decreasing systemic C5 levels to less than 1 percent of normal by week two, intravenous eculizumab didn’t significantly slow GA growth rates in patients with GA at either the six-month endpoint or after an additional six months of follow-up.29
Another C5 inhibitor, avacincaptad pegol (Zimura, Iveric Bio, New York, New York), met its prespecified primary endpoint of reducing the rate of GA in a randomized, controlled Phase IIb clinical trial. The reduction in the mean rate of GA growth over a year was 27.38 percent (p=0.0072) for the 2-mg group compared to sham. In the 4-mg group, the percentage was 27.81 percent (p=0.0051).30
Lampalizumab (Genentech, San Francisco) is an antigen-binding fragment (Fab) of a humanized monoclonal antibody that acts as a selective inhibitor of complement factor D, the rate-limiting enzyme in the alternative pathway.31 The Phase II (MAHALO) trial of lampalizumab for geographic atrophy secondary to age-related macular degeneration suggested that it could reduce the rate of GA enlargement.32 This led to twin Phase III trials, SPECTRI and CHROMA, that were identical, double-masked, randomized trials to evaluate the efficacy and safety of 10 mg of lampalizumab administered every four or six weeks by intravitreal injection, versus sham injections.33,34 Additionally, both trials were designed to assess whether having the CFI biomarker influences the treatment response. Together, SPECTRI and CHROMA enrolled more than 1,800 participants in more than 20 countries. However, lampalizumab didn’t appear to slow lesion progression and there was no link between faster GA progression and the presence of the CFI biomarker.33
Pegcetacoplan (APL-2; Apellis Pharmaceuticals, Waltham, Massachusetts) is a synthetic molecule that selectively inhibits C3, effectively downregulating all three complement pathways. APL-2 was evaluated in a Phase II trial (FILLY), which enrolled 246 subjects from 43 clinical sites internationally. Subjects were randomly assigned (2:2:1:1) to four arms: 15 mg APL-2 monthly (n=86); 15 mg APL-2 every other month (EOM; n=79); sham monthly (n=41); and sham EOM (n=40). The total treatment period was 12 months followed by a six-month observation period. The 12-month primary efficacy outcome was the difference in mean change from baseline GA area based on fundus autofluorescence (FAF). Secondary endpoints included the change in BCVA and the incidence of conversion to exudative AMD, as well as other safety endpoints.
At 12 months, patients who received intravitreal APL-2 every month had a 29 percent lower rate of GA lesion growth compared with sham (p=0.008), while patients who received APL-2 EOM had a 20 percent lower rate (p=0.067). The effect was most prominent in the second six months of treatment, during which monthly and EOM APL-2 slowed GA growth by 47 percent (p<0.001) and 33 percent (p=0.01), respectively, compared with sham.35 No differences in BCVA outcomes were observed between the groups.
Adverse events included endoph-thalmitis in two participants (2.3 percent) in the monthly group and one patient (1.3 percent) in the every-other-month group. There was an increased incidence of exudation in APL-2-treated eyes (20.9 percent in the monthly group and 8.9 percent in the EOM group) compared to sham-treated eyes (1.2 percent), which was responsive to standard-of-care treatment. Among patients with a history of CNV in the fellow eye, 13 of 36 (36.1 percent) in the monthly group, five of 28 (17.9 percent) in the EOM group, and none in the sham group developed exudative AMD. For those with no history of CNV in the fellow eye, exudative AMD developed in five of 50 (10 percent) and two of 51 (3.9 percent) in the monthly and EOM groups, respectively, and in one of 52 (1.9 percent) in the sham group. Two 30-month, Phase III, multicenter, randomized, double-masked, sham-injection controlled clinical trials (DERBY and OAKS) are currently under way. The two trials will enroll 600 patients internationally across four study arms: APL-2 15 mg monthly for 24 months; APL-2 15 mg EOM for 24 months; and two respective sham groups.36
As the preceding review shows, unlike wet AMD, an effective pharmacologic treatment for GA secondary to dry AMD remains elusive. To date, investigational agents have failed to meet study endpoints in either Phase II or III trials. However, recent and ongoing trials have demonstrated some promise. Therefore, despite past failures there’s reason to remain optimistic that a safe and effective treatment is still on the horizon. REVIEW
Dr. Ammar is a vitreoretinal surgery Fellow at the Wills Eye Hospital Retina Service. He has no financial interest in any of the products mentioned.
Dr. Chiang practices at the Wills Eye Hospital Retina Service and Mid Atlantic Retina. He’s an assistant professor of ophthalmology at Thomas Jefferson University. He’s received research grant support from Genentech, Regeneron and Apellis, and is a consultant for Orbit Biomedical/Gyroscope Therapeutics, Recens Medical and Apellis.
1. Ferris FL. Senile macular degeneration: Review of epidemiologic features. Am J Epidemiol 1983;118:2:132-151.
2. Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic Atrophy: Clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:5:1079-1091.
3. Chakravarthy U, Wong TY, Fletcher A, Piault E, Evans C, Zlateva G, Buggage R, Pleil A, Mitchell P. Clinical risk factors for age-related macular degeneration: a systematic review and meta-analysis. BMC Ophthalmol. 2010;10:31.
4. Bavik C, Henry SH, Zhang Y, Mitts K, McGinn T, Budzynski E, Pashko A, Lieu KL, Zhong S, Blumberg B, Kuksa V, Orme M, Scott I, Fawzi A, Kubota R. Visual cycle modulation as an approach toward preservation of retinal integrity. PLOS ONE. 2015;10:5:e0124940. doi:10.1371/journal.pone.0124940.
5. Rosenfeld PJ, Dugel PU, Holz FG, Heier JS, Pearlman JA, Novack RL, Csaky KG, Koester JM, Gregory JK, Kubota R. Emixustat hydrochloride for geographic atrophy secondary to age-related macular degeneration: A randomized clinical trial. Ophthalmology 2018;125:10:1556-1567.
6. Phase 3 Study of ALK-001 in Geographic Atrophy.
ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03845582. Accessed November 30, 2019.
7. WoldeMussie E, Ruiz G, Wijono M, Wheeler LA. Neuroprotection of retinal ganglion cells by brimonidine in rats with laser-induced chronic ocular hypertension. Invest Ophthalmol Vis Sci 2001;42:12:2849-2855.
8. Sena DF, Lindsley K. Neuroprotection for treatment of glaucoma in adults. Cochrane Database Syst Rev 2017;1:CD006539. doi:10.1002/14651858.CD006539.pub4.
9. A Safety and Efficacy Study of Brimonidine Intravitreal Implant in Geographic Atrophy Secondary to Age-related Macular Degeneration. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02087085. Accessed January 10, 2020.
10. Lund RD, Wang S, Lu B, Girman S, Holmes T, Sauvé Y, Messina DJ, Harris IR, Kihm AJ, Harmon AM, Chin F-Y, Gosiewska A, Mistry SK. Cells isolated from umbilical cord tissue rescue photoreceptors and visual functions in a rodent model of retinal disease. Stem Cells 2007;25:3:602-611.
11. A Safety Study of CNTO 2476 in Patients With Age-Related Macular Degeneration. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01226628. Accessed 10 January 2020.
12. Ho AC, Chang TS, Samuel M, Williamson P, Willenbucher RF, Malone T. Experience with a subretinal cell-based therapy in patients with geographic atrophy secondary to age-related macular degeneration. American Journal of Ophthalmology 2017;179:67-80.
13. A Study of an Encapsulated Cell Technology (ECT) Implant for Patients With Atrophic Macular Degeneration. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT00447954. Accessed 10 January 10 2020.
14. Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman J-P, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. The Lancet 2015;385:9967:509-516.
15. Kashani AH, Lebkowski JS, Rahhal FM, Avery RL, Salehi-Had H, Dang W, Lin C-M, Mitra D, Zhu D, Thomas BB, Hikita ST, Pennington BO, Johnson LV, Clegg DO, Hinton DR, Humayun MS. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med 2018;10:435.
16. Kashani AH, Uang J, Mert M, Rahhal F, Chan C, Avery RL, Dugel P, Chen S, Lebkowski J, Clegg DO, Hinton DR, Humayun MS. Surgical method for implantation of a biosynthetic retinal pigment epithelium monolayer for geographic atrophy: Experience from a Phase I/IIa study. Oph Retina. 2019;0:0.
17. Study of Subretinal Implantation of Human Embryonic Stem Cell-Derived RPE Cells in Advanced Dry AMD. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02590692. Accessed 30 November 2019.
18. da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, Vernon A, Daniels JT, Nommiste B, Hasan SM, Gooljar SB, Carr A-JF, Vugler A, Ramsden CM, Bictash M, Fenster M, Steer J, Harbinson T, Wilbrey A, Tufail A, Feng G, Whitlock M, Robson AG, Holder GE, Sagoo MS, Loudon PT, Whiting P, Coffey PJ. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol 2018;36;4:328-337.
19. Retinal Pigment Epithelium Safety Study For Patients In B4711001. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03102138. Accessed January 12, 2020.
20. Alikhan A, Kurek L, Feldman SR. The role of tetracyclines in rosacea. Am J Clin Dermatol 2010;11;2:79-87.
21. Minocycline block copolymer micelles and their anti-inflammatory effects on microglia. PubMed-NCBI. https://www-ncbi-nlm-nih-gov.proxy1.lib.tju.edu/pubmed/19937662. Accessed 12 January 2020.
22. Clinical Study to Evaluate Treatment With Oracea for Geographic Atrophy (TOGA). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01782989. Accessed 1 December 2019.
23. DeWan A, Liu M, Hartman S, Zhang SS-M, Liu DTL, Zhao C, Tam POS, Chan WM, Lam DSC, Snyder M, Barnstable C, Pang CP, Hoh J. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science 2006;314;5801:989-992.
24. A Study Assessing the Safety, Tolerability, and Efficacy of RO7171009 in Participants With Geographic Atrophy Secondary to Age-Related Macular Degeneration (AMD). ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03972709. Accessed 2 December 2 2019.
25. Khandhadia S, Cipriani V, Yates JRW, Lotery AJ. Age-related macular degeneration and the complement system. Immunobiology 2012;217;2:127-146.
26. Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: Genetics and biology coming together. Annual Review of Genomics and Human Genetics 2014;15:1:151-171.
27. Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Bowes Rickman C, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Progress in Retinal and Eye Research 2010;29:2:95-112.
28. Sarma JV, Ward PA. The complement system. Cell Tissue Res 2011;343:1:227-235.
29. Yehoshua Z, Alexandre de Amorim Garcia Filho C, Nunes RP, Gregori G, Penha FM, Moshfeghi AA, Zhang K, Sadda S, Feuer W, Rosenfeld PJ. Systemic complement inhibition with eculizumab for geographic atrophy in age-related macular degeneration: The COMPLETE study. Ophthalmology 2014;121:3:693-701.
30. Zimura in Subjects With Geographic Atrophy Secondary to Dry Age-Related Macular Degeneration. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02686658. Accessed 1 December 2019.
31. Lesavre PH, Müller-Eberhard HJ. Mechanism of action of factor D of the alternative complement pathway. J Exp Med 1978;148:6:1498-1509.
32. Yaspan BL, Williams DF, Holz FG, Regillo CD, Li Z, Dressen A, van Lookeren Campagne M, Le KN, Graham RR, Beres T, Bhangale TR, Honigberg LA, Smith A, Henry EC, Ho C, Strauss EC, MAHALO Study Investigators. Targeting factor D of the alternative complement pathway reduces geographic atrophy progression secondary to age-related macular degeneration. Sci Transl Med 2017;9:395.
33. Holz FG, Sadda SR, Busbee B, Chew EY, Mitchell P, Tufail A, Brittain C, Ferrara D, Gray S, Honigberg L, Martin J, Tong B, Ehrlich JS, Bressler NM. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: Chroma and Spectri Phase 3 randomized clinical trials. JAMA Ophthalmol 2018;136:6:666-677. doi:10.1001/jamaophthalmol.2018.1544.
34. A Study Investigating the Safety and Efficacy of Lampalizumab Intravitreal Injections in Participants With Geographic Atrophy Secondary to Age-Related Macular Degeneration. ClinicalTrials.gov.
https://clinicaltrials.gov/ct2/show/NCT02247531.
Accessed 5 February 2020.
35. Singh RP. FILLY APL-2 (pegcetacoplan) geographic atrophy preliminary 18-month results. Presented at: Macula Society annual meeting; Feb. 21-24, 2018; Beverly Hills, California.
36. Study to Compare the Efficacy and Safety of Intravitreal APL-2 Therapy With Sham Injections in Patients With Geographic Atrophy (GA) Secondary to Age-Related Macular Degeneration. ClinicalTrials.gov.
https://clinicaltrials.gov/ct2/show/record/NCT03525600. Accessed 1 December 2019.