Prostaglandin analogs are currently the most widely used first-line glaucoma medications in the
One reason the answer isn't immediately obvious is that the medications most commonly chosen to add to a prostaglandin—beta-blockers (usually timolol), alpha-2 agonists (usually brimonidine) and carbonic anhydrase inhibitors (such as dorzolamide and brinzolamide)—don't lower intraocular pressure as much when used as an adjunct to a prostaglandin as they would if they were used by themselves. For example, when timolol is used as monotherapy we can expect a 25-percent reduction in IOP, on average. But used as an add-on therapy for a patient who's already on a prostaglandin, we typically see a substantially smaller additional reduction in IOP.
 With that in mind, our group recently completed a meta-analysis of multiple studies from the existing clinical trial literature, with the intention of shedding light on which medications might be most effective as an add-on to a prostaglandin when additional pressure reduction is needed.1 Here, I'd like to discuss some of what we learned from that meta-analysis and offer some suggestions about how to proceed when you find yourself in this situation.Comparing Pressure Reduction
With that in mind, our group recently completed a meta-analysis of multiple studies from the existing clinical trial literature, with the intention of shedding light on which medications might be most effective as an add-on to a prostaglandin when additional pressure reduction is needed.1 Here, I'd like to discuss some of what we learned from that meta-analysis and offer some suggestions about how to proceed when you find yourself in this situation.Comparing Pressure Reduction
Our study looked at data from all of the published clinical trials in which one of these adjunctive agents was added to a PGA in a masked, randomized fashion, usually with the purpose of comparing it to some other agent. We only included studies that reported both the baseline IOP with the patient on the prostaglandin analog alone and the IOP after the second agent was added. (Very few of the studies looked at nocturnal IOP, so we excluded those time points.) In the case of beta-blockers, they could be dosed either once or twice daily, whereas with alpha-2 agonists and carbonic anhydrase inhibitors, they could be dosed either b.i.d. (as is commonly done in clinical practice) or t.i.d. Both of those classes of agents are FDA-approved for t.i.d. dosing—unless they are part of a fixed combination with timolol, in which case they are FDA-approved for b.i.d. dosing.
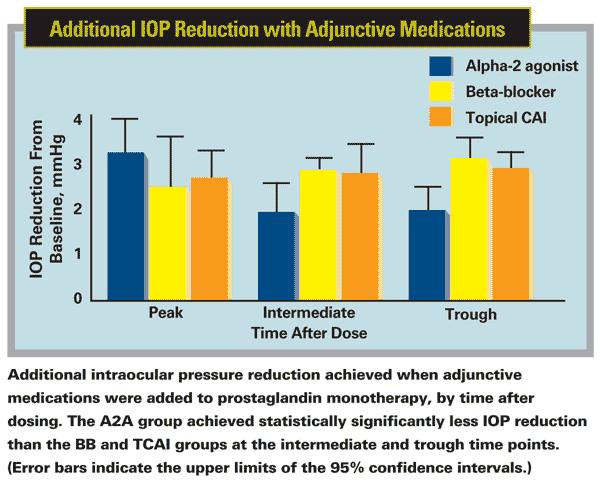
We extracted information from each clinical trial, categorized by the time after the last dose of the add-on agent. We considered the period one to four hours after the instillation of the add-on agent to be the period of peak, maximum effect. We also looked at the period more than four hours but less than nine hours after dosing, which we called the intermediate period. We referred to IOP measured more than nine hours after dosing as trough, or minimum-effect data.
The analysis showed no significant difference in the overall mean diurnal IOP reduction among the three classes of medication when used as an add-on to a prostaglandin. Furthermore, all three medications lowered IOP similarly during the peak period. However, we did find that the alpha-2 agonists were less effective than CAIs and beta-blockers at both trough and intermediate time points. It's notable that alpha-2 agonists have been shown to be more effective when dosed t.i.d. than b.i.d.2 (Interestingly, CAIs are also approved for t.i.d. use; however, one study found no statistically significant difference in effectiveness whether CAIs were used two or three times a day.3)
 This raises a question that we didn't address in our meta-analysis: What about the nocturnal period? A series of important reports from the
This raises a question that we didn't address in our meta-analysis: What about the nocturnal period? A series of important reports from the
Lowering IOP during the nocturnal period may be very important because studies have shown that IOP is highest during this time, as long as it's measured with the patient in what has been termed the habitual position, i.e., supine during the nocturnal period and sitting during the diurnal period. In this respect, CAIs, as adjunctive therapy, may have a significant treatment advantage over beta-blockers and alpha-2 agonists.6
Side Effects
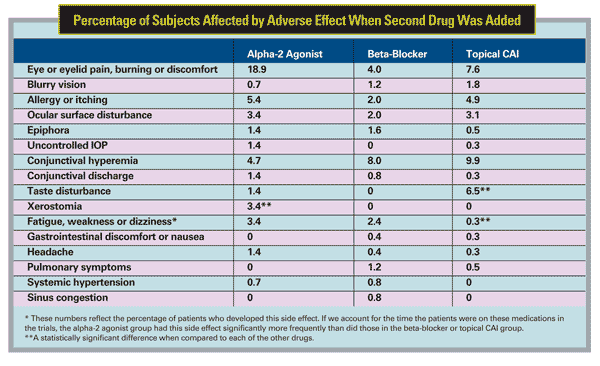
Our analysis also revealed that side-effect profiles vary substantially from class to class when these agents are added to a prostaglandin:
- Patients who received brinzolamide as the add-on complained of taste disturbance significantly more frequently than did patients in the other two groups.
- Significantly more patients receiving alpha-2 agonists reported eye or eyelid pain, burning or discomfort and dry mouth.
- Patients who received a beta-blocker or alpha-2 agonist were significantly more likely to complain of fatigue, weakness or dizziness than those in the CAI group. However, that result was based on analyzing the percentage of patients on a particular medication who developed this side effect. When we took into account the amount of time that the patients were on these medications in the trials, we found that the alpha-2 agonist group had this side effect significantly more frequently than did those in the beta-blocker or CAI group.
Although side effects like these are always a concern, at a practical level the reality is that most of these medications are well-tolerated by the majority of patients. In my experience, the key to managing potential side effects is to alert the patient ahead of time to the most common side effects associated with the medications you're prescribing, and to inform him of the possibility of switching to an alternate agent if it becomes necessary. Patients may find certain side effects, such as conjunctival hyperemia, dry mouth or taste disturbance easier to tolerate if they've been forewarned. Furthermore, if you don't mention the side effects in advance, your credibility is in jeopardy.
Other Alternatives
There are a few other possibilities that are worth considering when a prostaglandin doesn't lower pressure sufficiently:
• Switching prostaglandins. As you know, adding a second prostaglandin is out of the question because when prostaglandins are dosed twice a day or combined, the result tends to be an increase in IOP. However, in some individuals, one particular prostaglandin may be more effective than another. A previous report showed that there are receptor variants among individuals for prostaglandin analogs, which may explain why one prostaglandin could work better than another in a particular patient.7
Switching prostaglandins is somewhat controversial. Some might argue that a poor response to one prostaglandin, followed by a favorable response after switching to another, could represent a change in compliance; but I think most of us have observed that in some patients, one PGA seems more effective than another.
• Adding a fixed-combination product. This may seem like a good option, in that it's an easy way to get patients onto so-called maximal medical therapy. The problem is that adding a fixed-combination drug to a prostaglandin bypasses the important step of making sure the two medications you're adding are each beneficial for the patient. You're giving the patient the burden of the toxicity, side effects and expense of both medications without verifying that each one is giving the patient a beneficial IOP reduction. Furthermore, the law of diminishing returns begins to kick in when you're adding a second medication, and this effect is likely to be even more of a problem by the time you add a third agent.
In my opinion, the only time to use a fixed-combination product—except during an acute IOP crisis—is when you've made sure that both agents are of benefit to the patient. In that case, they are very helpful, especially in simplifying the drop regimen for the patient and, thereby, possibly improving compliance.
• Laser trabeculoplasty. This is a very reasonable option to consider (assuming it's not contraindicated). In some cases, ALT or SLT may even be effective enough to serve as the first and only initial therapy, so I also offer laser trabeculoplasty to many patients as a first-line option. In my experience, however, most patients opt for medical therapy. Patients tend to be a little bit timid about laser. It helps to explain that laser trabeculoplasty does not involve making holes in the tissue being treated, but rather that it triggers a biological response that ultimately results in IOP reduction in most patients.
Choosing an Add-On
If you're adding a second IOP-lowering agent to a prostaglandin, which one makes the most sense? Currently, I believe most ophthalmologists in this situation would choose to add a beta-blocker first. There are at least two good reasons for making this choice: convenient dosing and low cost. A patient can use a beta-blocker once a day in the morning, which is easier than having to instill a drop twice a day. However, given that beta -blockers don't appear to lower nocturnal IOP—when pressure may be at its highest—it seems to me we're losing an important therapeutic benefit by putting the patient on a beta-blocker in exchange for the convenience of q.d. dosing.
All things being equal, and assuming the patient favors additional medical therapy over laser trabeculoplasty, in this situation I'll choose the medication that lowers the pressure the most and has the least bothersome side effects. Based on what we learned from our study, I think a CAI makes the most sense. It has superior nocturnal pressure lowering efficacy; it seems to work well when used b.i.d.; during the day it provides a relatively stable pressure reduction compared to what you get with adding an alpha-2 agonist (which we found to be less effective during the intermediate and trough periods); and CAIs appear to be least offensive in terms of the likelihood of producing undesirable side effects.
So, if a prostaglandin isn't getting your patient's pressure low enough, my first suggestion for an add-on would be a CAI, barring a medical contraindication. The evidence suggests that your patient will derive the most pressure-lowering benefit from a CAI, with the least chance of side effects. That should increase the odds that your patient will do well in the long run, while leaving you with a happier patient in the meantime.
Dr. Tanna is vice chair of ophthalmology and director of the Glaucoma Service at the Feinberg School of Medicine at
1. Tanna AP, Rademaker AW, Stewart WC, Feldman RM. Meta-analysis of the Efficacy and Safety of a2-Adrenergic Agonists, b-Adrenergic
Antagonists, and Topical Carbonic Anhydrase Inhibitors With Prostaglandin Analogs. Arch Ophthalmol 2010;128:7:825-33.
2. Konstas AG, Stewart WC, Topouzis F, Tersis I, Holmes KT, Stangos NT. Brimonidine 0.2% given two or three times daily versus timolol maleate 0.5% inprimary open-angle glaucoma. Am J Ophthalmol 2001;131:6:729-733.
3. Silver LH. Clinical efficacy and safety of brinzolamide (Azopt), a new topical carbonic anhydrase inhibitor for primary open-angle glaucoma and ocular hypertension. Brinzolamide Primary Therapy Study Group. Am J Ophthalmol 1998;126:3:400-8.
4. Liu JH, Kripke DF, Weinreb RN. Comparison of the nocturnal effects of once-daily timolol and latanoprost on intraocular pressure. Am J Ophthalmol 2004;138:3:389-95.
5. Liu JH, Medeiros FA, Slight JR, Weinreb RN. Comparing diurnal and nocturnal effects of brinzolamide and timolol on intraocular pressure in patients receiving latanoprost monotherapy. Ophthalmology 2009;116:3:449-54. Epub 2009 Jan 20.
6. Liu JH, Medeiros FA, Slight JR, Weinreb RN. Diurnal and Nocturnal Effects of Brimonidine Monotherapy on Intraocular Pressure. Ophthalmology 2010 Jul 20. [Epub ahead of print]
7. Sakurai M, Higashide T, Takahashi M, Sugiyama K. Association between genetic polymorphisms of the prostaglandin F2alpha receptor gene and response to latanoprost. Ophthalmology 2007;114:6:1039-45. Epub 2007 Apr 30.



