Presentation
A 33-year-old African-American male presented to the Wills Eye Emergency Room with one month of right-eye redness, pain, floaters and blurred vision. He had initially been seen at another hospital where a prior history of trauma to the left eye had been elicited and a diagnosis of sympathetic ophthalmia was entertained.
Medical History
The patient had a history of anemia, peptic ulcer disease, high blood pressure and pneumonia requiring hospitalization two years prior to presentation. The patient denied a history of severe trauma. He had experienced slow, painless vision loss in the left eye five years prior to presentation. On review of systems, the patient denied fevers, shortness of breath and malaise, but endorsed chills, night sweats, a 30-pound weight loss over one year, as well as a cough. He also reported an old rash on his shins. The patient denied tobacco, alcohol or illicit drug use. He was incarcerated at the time of his presentation.
Examination
Uncorrected distance visual acuity in the right eye was 20/150 with no improvement with pinhole. The left eye had no light perception. The right pupil was irregular and the left was difficult to assess. Applanation tonometry was 12 mmHg in both eyes. Slit lamp examination of the right eye revealed moderate injection of the conjunctiva, fine keratic precipitates, 15 to 20 white blood cells per high-powered field in the anterior chamber, as well as moderate flare. There was no hypopyon. Examination of the iris revealed posterior synechiae. The lens was clear. The left eye was pre-phthisical, but quiet. On dilated fundus examination, the view to the posterior pole was quite hazy due to vitritis with vitreous "snowballs." Additionally, several areas of retinal whitening were seen (See Figure 1). There was no view possible in the left eye.
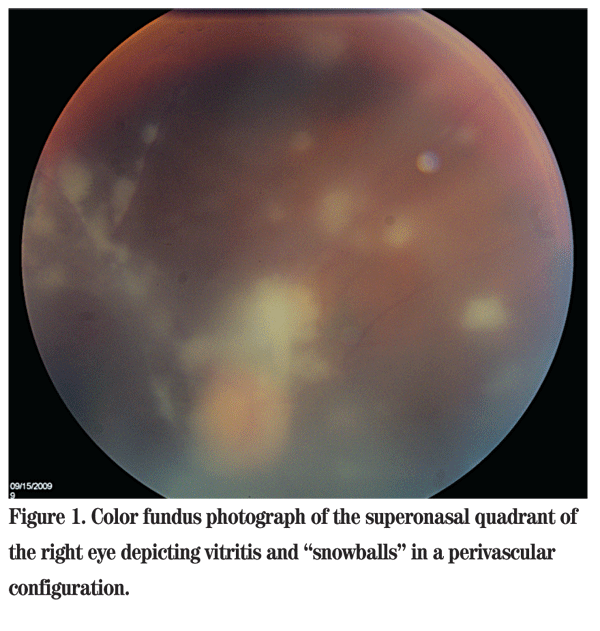
Diagnosis, Workup and Treatment
Due to his multiple laboratory abnormalities and a stable eye exam, the patient was transferred to the internal medicine service for further workup and management. At this point, records from his prior pneumonia episode were obtained. Two years prior the patient had been hospitalized at an outside institution, and had undergone bronchoscopy, which demonstrated non-caseating granulomata negative for acid-fast bacteria and fungus. Cultures for the same organisms were also negative and a diagnosis of sarcoidosis was made and the patient had been discharged on oral steroids. The patient had also been diagnosed with a severe cardiomyopathy with an ejection fraction estimated at 20 percent.
By the third hospital day, the pa-tient's visual acuity was 20/70. Results from PPD, RPR, HIV, ANA, HCV, C3, C4, Bartonella, Histoplasma, Anti-GBM became available and were all negative. Given this additional history and physical evidence, a diagnosis of sarcoidosis was made. The valacyclovir was stopped and oral prednisone was initiated at a dose of 60 mg by mouth daily. At this time, the patient's renal function was still impaired, though his hypercalcemia had improved. He underwent kidney biopsy, which revealed non-caseating granulomata and deposits of IgA (See Figure 2).
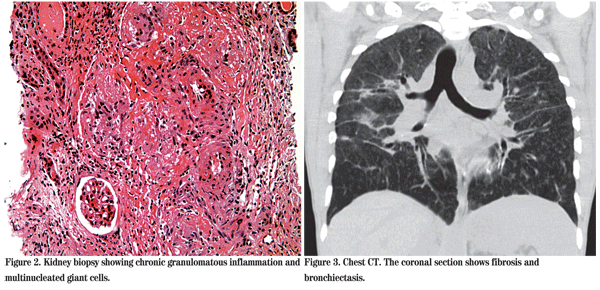
Later in the first hospital week, the results from further blood tests became available. The Angiotensin-converting Enzyme level was high at 356 (reference range: 9 to 67). Serum protein electrophoresis revealed an abnormal immunoglobulinemia that was later interpreted as polyclonal. Additionally, c-ANCA was positive (titer 1:80), as was Lyme antibody at 3.19 (range 0 to 0.90), and Rheumatoid Factor at 57 (range 0 to 30). These final three results were interpreted as false positive results in the setting of a severe chronic inflammation. CT examination of the chest demonstrated bronchiectasis and fibrosis consistent with stage 4 sarcoidosis (See Figure 3).
MRI of the brain showed no signs of CNS vasculitis. Due to his paraproteine-mia and anemia, a bone marrow biopsy was obtained, which again demonstrated non-caseating granulomata. At discharge his medical condition and vision had stabilized and prednisolone was reduced to every two hours, and he was maintained on prednisone 60 mg by mouth daily.
At three weeks follow-up, the vision was still 20/70, and the view to the posterior pole was significantly clearer and demonstrated the perivascular granulomata characteristic of sarcoidosis termed "taches de bougie" (candle-wax drippings). An epiretinal membrane was also present over the macula (See Figure 4). His prednisolone drops were reduced to four times daily, and he was pursuing follow-up with Rheumatology for further management of his immune suppression.

Discussion
An interesting feature of the present case are the white perivascular lesions that were more obvious on follow-up examination, but also visible at the initial presentation. The first description of what is now called "taches de bougie" comes from Frank Walsh in 1942.2 He described a case of a young woman who was thought to have ocular tuberculosis and a hopelessly affected left eye. After having been lost to follow-up for 10 years, she presented with "whiteish yellow masses" in a "perivascular situation" in the retina. The left eye was enucleated and "typical sarcoid tumors" were demonstrated. J. Donald Gass in 1976 demonstrated that these lesions seen by ophthalmoscopy reflect perivascular inflammation and subpigment epithelial granulomata in the case of a patient with sarcoidosis who died unexpectedly shortly after an ophthalmic exam.3 This localization in the retina has also been demonstrated more recently by high-resolution optical coherence tomography.4 Sarcoidosis is also thought to cause focal choroidal granulomata.
Sarcoidosis can affect the eye and orbit in many ways.5-7 Orbital and lacrimal gland inflammation, conjunctival granulomata, anterior uveitis, posterior uveitis and choroiditis have all been described. The mainstay of treatment is topical or systemic corticosteroids. For persistent cases, steroid sparing agents are used.
A very broad differential was initially considered in this case. In an incarcerated patient with a prephthisical contralateral eye, tuberculosis, syphilis, fungal endophthalmitis, viral retinitis and sympathetic ophthalmia were all felt to be reasonable initial possibilities. Our priority was to expeditiously rule out severe infection, while managing his inflammation.
Once the pa-tient's vision stabilized and initial laboratory data as well as old records became available, we felt comfortable stopping antiviral therapy and starting oral corticosteroids. Given the significant involvement of several organ systems, it is likely that this patient will need long-term systemic immune suppression to manage his sarcoidosis. In the long term, his visual prognosis will be affected by the fibrosis and epiretinal membrane evident on his follow-up examinations.
The author would like to thank Marc Spirn, MD, of the Wills Eye Institute Retina Service, John Farber, MD, of the Thomas Jefferson University Hospital Department of Pathology, and Paul Baker, MD, a fellow in vitreoretinal surgery at the Wills Eye Institute.
1. Nielsen RH. Ocular Sarcoidosis. Arch Ophthalmol 1959;61:657-663.
2. Walsh FB. Ocular Importance of Sarcoid: Its Relation to Uveoparotid Fever. Archives of Ophthalmology 1939;21:421.
3. Gass JDM, Olson CL. Sarcoidosis With Optic Nerve and Retinal Involvement. Arch Ophthal 1976;94:945-950.
4. Wong M, Janowicz M, Tessler HH, Goldstein DA. High-resolution optical coherence tomography of presumed sarcoid retinal granulomas. Retina 2009;29:1545-1546.
5. Iannuzzi MC, Rybicki BA,
6. Bonfioli AA, Orefice F. Sarcoidosis. Semin Ophthalmol 2005;20:177-182.
7. Herbort CP, Rao NA, Mochizuki M. International criteria for the diagnosis of ocular sarcoidosis: Results of the first International Workshop On Ocular Sarcoidosis (IWOS). Ocul Immunol Inflamm 2009;17:160-169.



