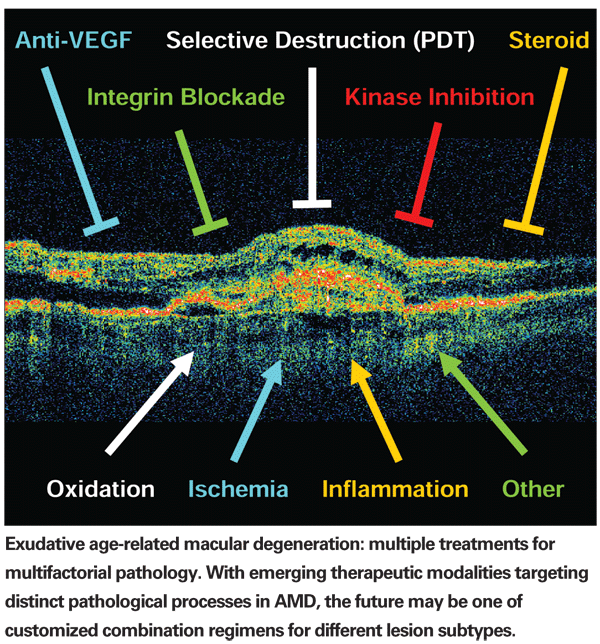
The combination of therapeutic modalities targeting different molecular pathways or different aspects of a pathological process is believed to enhance treatment efficacy through additive or synergistic effects. This principle is applied in the management of numerous pathological states, including systemic hypertension, diabetes mellitus, pain, infection and cancer.
In the management of exudative age-related macular degeneration, combination approaches are currently being investigated as a means of improving treatment efficacy as well as reducing treatment frequency. The concept of combination therapy for AMD has become possible only within the past decade as a result of the introduction of important treatment modalities such as verteporfin (Visudyne) photodynamic therapy and inhibitors of vascular endothelial growth factor such as pegaptanib (Macugen), ranibizumab (Lucentis) and bevacizumab (Avastin). Prior to the use of VEGF inhibitors for AMD, studies reported successful treatment using combinations of PDT and corticosteroids using various doses and delivery modalities.1 Improved outcomes and fewer ocular side effects with ranibizumab and bevacizumab, however, have led to the widespread use of these agents and their incorporation into investigational combination regimens in recent years. For this reason, this review will focus on combination regimens using anti-VEGF agents.
VEGF Inhibitors and PDT
In the Minimally Classic/ Occult Trial of the Anti-VEGF Antibody Ranibizumab in the Treatment of Neovascular AMD (MARINA) and the Anti-VEGF Antibody for the Treatment of Predominantly Classic Choroidal Neovascularization in AMD (ANCHOR) studies, evaluating the safety and efficacy of intravitreal ranibizumab for the treatment of neovascular AMD, intravitreal injections were administered on a fixed monthly basis for two years.2,3 Although the incidence of serious ocular adverse events such as endophthalmitis, severe uveitis and rhegmatogenous retinal detachment was low (<1.3 percent for any given adverse outcome in either of these studies), this level of injection-related risk is nevertheless significant. In addition, there appears to be a dose-related increase in stroke incidence with the use of ranibizumab.4 In practice, from the standpoints of both safety and cost, it is clearly desirable to limit the frequency and number of injections if one can do so without sacrificing efficacy. Thus, the possibility of reducing injection frequency by combining anti-VEGF agents with PDT for selected AMD cases is an appealing alternative to repeated injections, since combination regimens should theoretically be able to achieve this goal while maintaining or even improving efficacy.
The earliest data on PDT in combination with a VEGF inhibitor came from Phase II investigations with pegaptanib.5 Eleven of the subjects in this study had predominantly classic CNV and were treated with standard fluence PDT followed five to 10 days later by an intravitreal injection of 3 mg pegaptanib, which was repeated at 28-day intervals. At three months, 90 percent had stable vision and 60 percent had gained three or more lines.
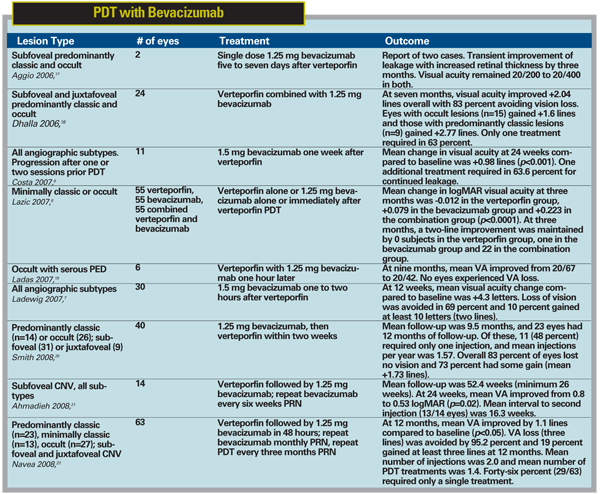
Combination therapy using PDT and bevacizumab was evaluated in a small, prospective, uncontrolled case series in Brazil and showed modest visual acuity improvement at six months in 11 subjects treated with standard fluence PDT followed one week later by intravitreal bevacizumab.6 Mean visual acuity improved from 20/200 at baseline to 20/160 at six months (p<0.001) with a mean gain of +0.98 lines. In another report on 30 subjects with neovascular AMD, bevacizumab administered one to two hours after PDT resulted in avoiding a loss of 15 letters of visual acuity in all subjects.7 At three months, 69 percent had unchanged or improved vision and 10 percent gained 10 or more letters.
Retinal edema and subretinal fluid on optical coherence tomography, and fluorescein angiographic leakage resolved in all subjects by three months.
In a larger, randomized, controlled trial, 165 subjects with minimally classic or occult CNV were randomly assigned to a single session of PDT, a single intravitreal injection of bevacizumab or a combination regimen in which bevacizumab was administered within one hour after PDT.8 At three months, visual acuity by logMAR had improved from baseline in the bevacizumab and combination groups but slightly declined in the PDT monotherapy group (+0.079, +0.223 and -0.012, respectively; p<0.0001). At one month, a greater than two-line gain in vision was observed in 30 percent, 56 percent and 2 percent of the subjects randomized to bevacizumab, combination and PDT monotherapy, respectively. At three months the percentage of subjects with greater than two lines of visual acuity gain (2 percent, 42 percent and 0 percent, respectively) had declined in all groups, but the decline was most pronounced in the bevacizumab monotherapy group.
Central retinal thickness as measured by OCT was decreased in all three treatment groups. Although it is not surprising that bevacizumab performed better than PDT monotherapy in minimally classic and occult lesions, it is noteworthy that the short-lived positive effect of bevacizumab monotherapy appeared to be extended in the combination therapy group, supporting the hypothesis that combination with PDT can lead to increased efficacy and reduced injection frequency. The results of some key case series evaluating the combination of PDT with bevacizumab are summarized in Table 1. Larger studies with longer follow-up are needed to confirm these findings, but these early data are encouraging.
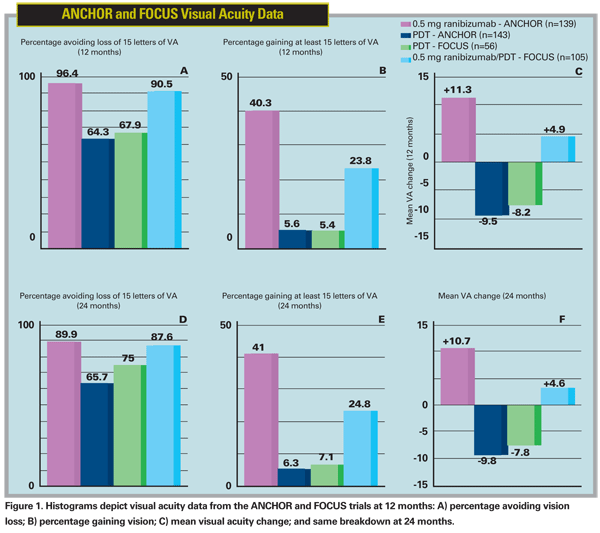
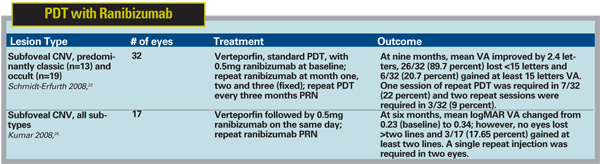
Similar data have been reported using ranibizumab in combination with PDT. The RhuFab V2 Ocular Treatment Combining the Use of Visudyne to Evaluate Safety (FOCUS) trial, a prospective, randomized, single-masked Phase II study, randomly assigned 162 subjects with predominantly classic subfoveal CNV to treatment with ranibizumab seven days after PDT or to PDT monotherapy.9 At 12 months, 90.5 percent of subjects in the combination group avoided the loss of 15 letters of visual acuity compared with 67.9 percent of subjects in the PDT monotherapy group (p<0.001). Also, 23.8 percent of subjects in the combination group gained at least 15 letters compared with 5.4 percent of subjects receiving PDT monotherapy. Combination therapy reduced the number of PDT sessions needed as 91.1 percent of subjects in the PDT monotherapy group required repeat treatments within the first 12 months compared with 27.6 percent of the combination therapy group (p<0.001). Similar results have been reported after the analysis of the two-year data from the FOCUS trial (See Figure 1).10 Thus, combination therapy was superior to PDT alone in efficacy and also reduced the need for repeat PDT sessions.
It is important to note that ranibizumab was given at fixed monthly doses and this study was not designed to compare combination therapy to ranibizumab monotherapy. There appear to be comparable levels of vision preservation in the combination group in the FOCUS study and the ranibizumab monotherapy group in the ANCHOR study,2 with more favorable results in the ANCHOR study with respect to visual acuity gain (See Figure 1). The comparison depicted here is solely for illustrative purposes—the two study populations had different baseline characteristics, including the fact that ANCHOR study subjects were treatment naïve, whereas almost half of the subjects in the FOCUS study had received prior treatments for AMD.
The
Triple Therapy
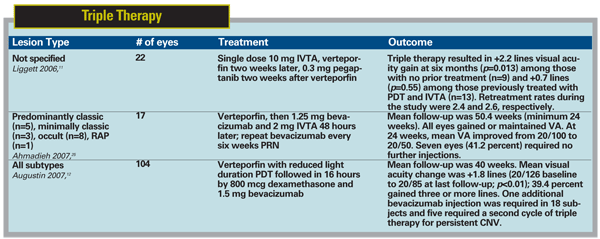
Combination therapy does not need to be limited to two modalities, and some investigators have suggested that the multifactorial nature of the pathogenesis of neovascular AMD may be better addressed by a combination of PDT with a corticosteroid as well as a VEGF inhibitor. In one retrospective case series, 22 eyes were treated with 10 mg of triamcinolone followed two weeks later by PDT and 0.3 mg of pegaptanib in another two weeks.11 In the nine eyes with no history of prior treatment, visual acuity improved by 2.2 lines at six months when compared to baseline (p=0.013), but among the 13 eyes with a history of prior treatment, only one (7.69 percent) achieved two lines of visual acuity improvement.
In a larger, prospective case series, the combination of PDT with bevacizumab and dexamethasone was found to be beneficial as well.12 In 104 subjects with CNV of all subtypes, PDT with reduced delivery time (70 seconds) was followed within 16 hours by 800 mcg of dexamethasone and 1.5 mg of bevacizumab after a monoport vitrectomy, which was reportedly performed to avoid drug reflux. Although there were methodological differences with other studies, this approach yielded similar results with respect to visual acuity. Compared to baseline, there was an increase of +1.8 lines of visual acuity for the population as a whole (p<0.01) at a mean follow-up of 40 weeks, with 96.2 percent of the subjects avoiding three lines of vision loss and 39.4 percent gaining at least three lines.
Noteworthy in the latter study is the attempt to reduce the PDT light dose (reportedly for increased safety) by decreasing the delivery time from the standard duration of 83 seconds to 70 seconds. This does not constitute reduced fluence PDT as defined in the VIM study, in which a reduction in total fluence was achieved by decreasing the laser fluence rate from 600 mW/cm2 to 300 mW/cm2;13 however, it is an alternative approach to accomplish the same goal of decreasing the total light dose in an effort to minimize possible dose-dependent damaging effects of PDT on normal choroid and RPE.14-16
Another way to decrease damage without altering light dose would be to inject less verteporfin. The concept of "PDT dose" will become an important aspect of future combination therapies incorporating PDT; however, it is important to recognize that these various approaches are almost certainly not equally effective. For instance, since the production of free radicals from circulating oxygen is a non-linear process, reducing delivery time by half would not be predicted to have the same effect as reducing fluence rate by half. At this time, it is not certain which approach is most effective; however, the method used in the VIM trial is certainly the most studied.
New Questions
With an ever-increasing understanding of the pathological processes involved in neovascular AMD and the continuing introduction of novel therapeutic modalities targeting different aspects of that pathology, combination approaches are expected to provide better treatments through improved efficacy while minimizing the risks associated with any single therapy. A number of small, early studies have begun to answer important questions about the efficacy and safety of combining PDT with intravitreal pharmacotherapy; however, many important new questions have also been raised by this work and will need to be addressed in studies over the next decade.
First, although the combination of PDT with anti-VEGF therapy, with or without corticosteroids, has been shown to be beneficial in the treatment of CNV, most of the studies to date have been either uncontrolled or have used PDT monotherapy as the control group. With the recognized superior efficacy of VEGF inhibitors, an important remaining question is whether the addition of PDT to the regimen will offer additional benefit. Many feel that combination with PDT will decrease the frequency of injections, but that the possibility of demonstrating better efficacy may be limited by a ceiling effect when compared to the excellent results already reported for VEGF inhibitor monotherapy.
Also, it is unclear whether certain angiographic lesion subtypes are more likely to benefit from combination therapy. Trials such as the
Second, if PDT is to be used in combination with other approaches, it will be important to develop a better understanding of the effects of various fluence modulations. The VIM trial showed that PDT with reduced fluence can be at least as effective as standard fluence. Whether reduced fluence indeed results in better long-term visual outcomes, what method is best for modulating fluence and how to determine the optimal fluence dose for a given lesion are important questions that future large trials will need to address.
Finally, it is known that the pathology of AMD is multifactorial and not all CNV respond in the same way to the same treatment. With the increasing use of OCT in the management of AMD, more refined prognostic criteria based on a combination of angiography and newer imaging modalities may more accurately predict the response of a given lesion to a given treatment. With increasing recognition of the role of genetics in AMD, genotyping may also provide important prognostic data in the future. With such information and a growing armamentarium of therapies targeting different pathological processes, the future management of neovascular AMD may employ customized multi-modality regimens.
The authors are at the Cole Eye Institute, Cleveland Clinic Foundation. Contact Dr. Kaiser at
1. Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis 2007;10:141-8.
2. Brown DM,
3. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419-31.
4. Dafer RM, Schneck M, Friberg TR, Jay WM. Intravitreal ranibizumab and bevacizumab: A review of risk. Semin Ophthalmol 2007;22:201-4.
5. Anti-vascular endothelial growth factor therapy for subfoveal choroidal neovascularization secondary to age-related macular degeneration: Phase II study results. Ophthalmology 2003;110:979-86.
6. Costa RA, Jorge R, Calucci D, Melo LA, Jr., Cardillo JA, Scott IU. Intravitreal bevacizumab (Avastin) in combination with verteporfin photodynamic therapy for choroidal neovascularization associated with age-related macular degeneration (IBeVe Study). Graefe's Arch Clin Exp Ophthalmol 2007;245:1273-80.
7. Ladewig MS, Karl SE, Hamelmann V, et al. Combined intravitreal bevacizumab and photodynamic therapy for neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2008;246:17-25.
8. Lazic R, Gabric N. Verteporfin therapy and intravitreal bevacizumab combined and alone in choroidal neovascularization due to age-related macular degeneration. Ophthalmology 2007;114:1179-85.
9. Heier JS, Boyer DS, Ciulla TA, et al. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration: Year 1 results of the FOCUS Study. Arch Ophthalmol 2006; 124:1532-42.
10. Antoszyk AN, Tuomi L, Chung CY, Singh A. Ranibizumab combined with verteporfin photodynamic therapy in neovascular age-related macular degeneration (FOCUS): year 2 results. Am J Ophthalmol 2008;145:862-74.
11. Liggett PE, Colina J, Chaudhry NA, Tom D, Haffner G. Triple therapy of intravitreal triamcinolone, photodynamic therapy, and pegaptanib sodium for choroidal neovascularization. Am J Ophthalmol 2006; 142:1072-4.
12. Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: Verteporfin PDT, bevacizumab, and dexamethasone. Retina 2007;27:133-40.
13. Azab M, Boyer DS,
14. Postelmans L, Pasteels B, Coquelet P, El Ouardighi H, Verougstraete C, Schmidt-Erfurth U. Severe pigment epithelial alterations in the treatment area following photodynamic therapy for classic choroidal neovascularization in young females. Am J Ophthalmol 2004;138:803-8.
15. Schlotzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe's Arch Clin Exp Ophthalmol 2002;240:748-57.
16. Wachtlin J, Behme T, Heimann H, Kellner U, Foerster MH. Concentric retinal pigment epithelium atrophy after a single photodynamic therapy. Graefe's Arch Clin Exp Ophthalmol 2003;241:518-21.
17. Aggio FB, Melo GB, Hofling-Lima AL, Eid Farah M. Photodynamic therapy with verteporfin combined with intravitreal injection of bevacizumab for exudative age-related macular degeneration. Acta Ophthalmol Scand 2006;84:831-3.
18. Dhalla MS, Shah GK, Blinder KJ, Ryan EH, Jr., Mittra RA, Tewari A. Combined photodynamic therapy with verteporfin and intravitreal bevacizumab for choroidal neovascularization in age-related macular degeneration. Retina 2006;26:988-93.
19. Ladas ID, Kotsolis AI, Papakostas TD, Rouvas AA, Karagiannis DA, Vergados I. Intravitreal bevacizumab combined with photodynamic therapy for the treatment of occult choroidal neovascularization associated with serous pigment epithelium detachment in age-related macular degeneration. Retina 2007;27: 891-6.
20. Smith BT, Dhalla MS, Shah GK, Blinder KJ, Ryan EH, Jr., Mittra RA. Intravitreal injection of bevacizumab combined with verteporfin photodynamic therapy for choroidal neovascularization in age-related macular degeneration. Retina 2008;28:675-81.
21. Ahmadieh H, Taei R, Soheilian M, Riazi-Esfahani M, Ahadi H. Single-session photodynamic therapy combined with intravitreal bevacizumab for neovascular age-related macular degeneration. Eur J Ophthalmol 2008;18:297-300.
22. Navea A, Mataix J, Desco MC, Garcia-Pous M, Palacios E. One-Year Follow-up of Combined Customized Therapy. Photodynamic Therapy and Bevacizumab for Exudative Age-Related Macular Degeneration. Retina 2008. [Epub ahead of print]
23. Schmidt-Erfurth U, Wolf S. Same-day administration of verteporfin and ranibizumab 0.5 mg in patients with choroidal neovascularisation due to age-related macular degeneration. Br J Ophthalmol 2008;92:1628-1635.
24. Kumar A, Gopalakrishnan K, Sinha S. Combination photodynamic therapy and intravitreal ranibizumab in neovascular AMD in a north Indian population: A pilot study. Retina 2008;28:1132-7.
25. Ahmadieh H, Taei R, Soheilian M, et al. Single-session photodynamic therapy combined with intravitreal bevacizumab and triamcinolone for neovascular age-related macular degeneration. BMC Ophthalmol 2007;7:10



