Presentation
A 27-year-old female was referred to a local ophthalmologist with recurrent unilateral uveitis of her left eye. She had a one-year history of recurrent episodes of transient vision loss OS. Evaluation revealed elevated intraocular pressure of 56 mmHg and anterior chamber inflammation OS, for which she was treated with brimonidine twice a day, dorzolamide-timolol twice a day, netarsudil-latanoprost at bedtime nightly, acetazolamide 500 mg twice a day by mouth, and prednisolone acetate eye drops four times a day. She was also started on oral valacyclovir 1 gram three times daily due to concern for a herpetic infection.
At one-month follow-up, there was persistent anterior chamber inflammation with granulomatous keratic precipitates and a fixed pupil OS. Difluprednate was substituted, and she underwent an anterior chamber paracentesis, which was negative for varicella zoster virus, herpes simplex virus and cytomegalovirus. At six-months follow-up, IOP OS remained above 30 mmHg despite maximal medical therapy, so an Ahmed tube shunt was placed superotemporally. However, IOP remained elevated in the range of 30 to 50 mmHg, which was attributed to corticosteroid response, so topical corticosteroids were discontinued. Intraocular inflammation worsened, so difluprednate twice a day was restarted, and acyclovir was switched to ganciclovir four times a day.
The patient was referred to a uveitis specialist, who diagnosed intraocular malignancy, based on clinical examination and ultrasound biomicroscopic findings, and the patient was referred to the Ocular Oncology Service at the Wills Eye Hospital for biopsy and management.
An extensive uveitis workup prior to presentation was negative for tuberculosis, syphilis, angiotensin-converting enzyme, lysozyme and HLA-B27. A chest computerized tomography scan showed calcified and noncalcified granulomas in the left lower lobe and densely calcified mediastinal and hilar nodes, which were interpreted as possible sequelae of prior histoplasmosis infection.
Medical History
 |
The patient’s past medical history revealed hypothyroidism and migraine headaches without ocular involvement. Family history was remarkable for multiple sclerosis in her maternal aunt, breast cancer in her maternal grandmother and lung cancer in her maternal grandfather.
Exam
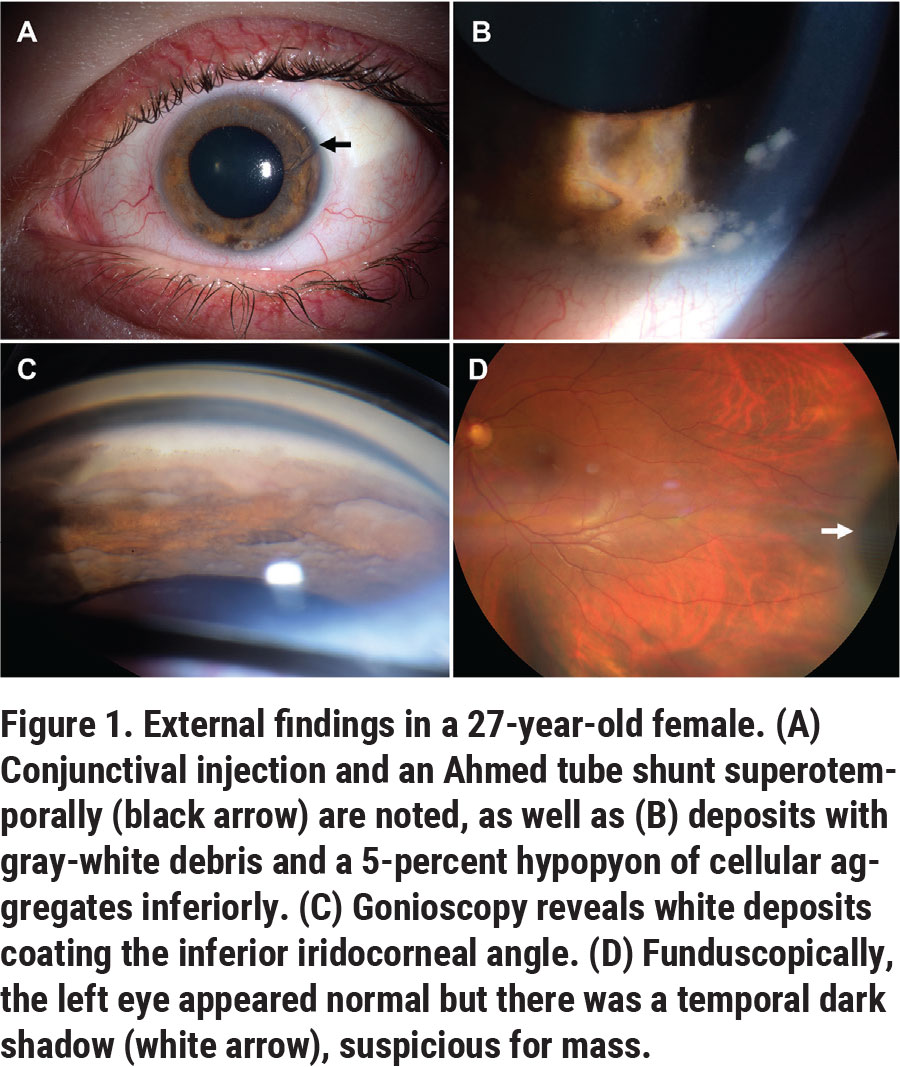
On examination, the patient’s best-corrected visual acuity was 20/20 OD and 20/40 OS. The pupils were 3 mm OD and 7 mm OS in the dark, with minimal reactivity to light and a relative afferent pupillary defect OS. The IOP was 17 mmHg OD, and 31 mmHg OS. Confrontation visual fields and extraocular movements were full. Slit lamp biomicroscopy OS revealed the Ahmed valve in place superotemporally (Figure 1A, arrow), gray-white deposits coating the corneal endothelium, iris stroma and angle (Figure 1B); and a 5-percent hypopyon composed of clumps of focally pigmented cells inferiorly (Figure 1C). Aggregates of cells also were noted in the lumen of the Ahmed tube shunt (Figure 1A, arrow). Gonioscopy revealed focal anterior synechiae and gray-white deposits blanketing the inferior angle and iris (Figure 1C). Dilated fundus examination disclosed a flat retina and intact macula, no vitreous cells and a normal optic disc. A dark shadow, suspicious for a mass, was noted in the far temporal periphery in the ciliary body (Figure 1D, white arrow). Examination of the right eye was unremarkable with no inflammation or tumor.
What is your diagnosis? What further workup would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
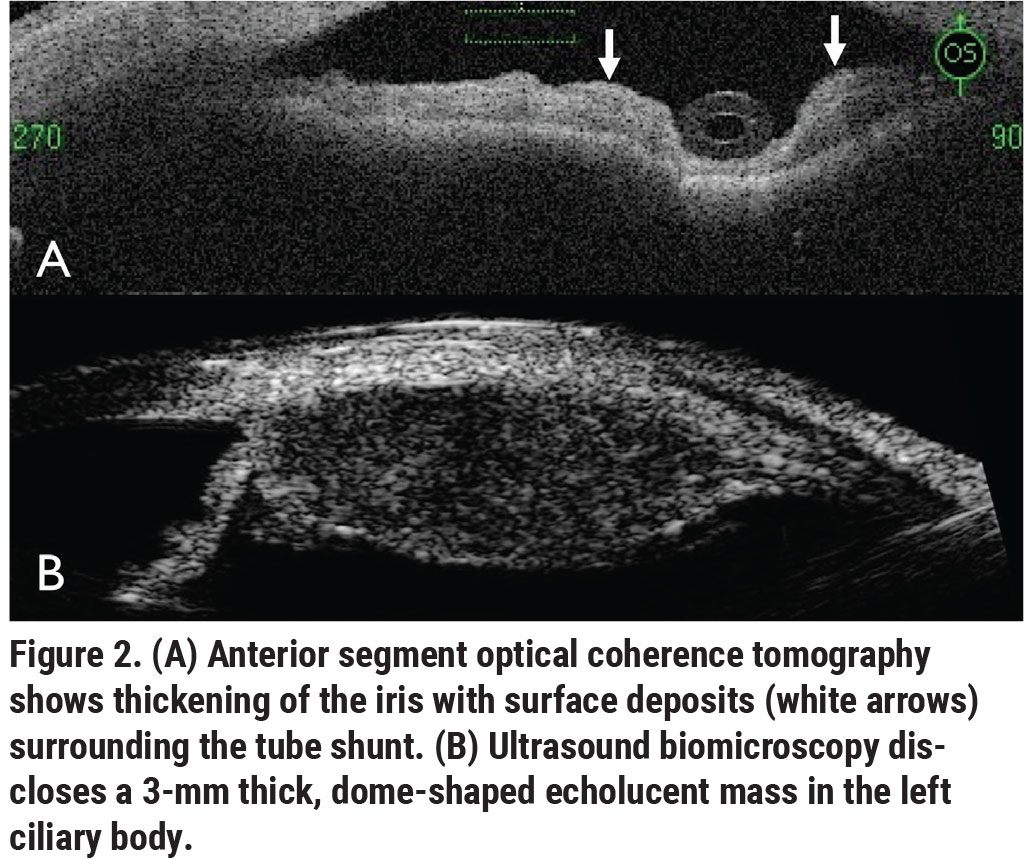 |
The clinical picture wasn’t consistent with infectious or non-infectious uveitis; instead, the clumps of cells coating the anterior chamber were only consistent with a malignancy. B-scan ultrasonography showed a flat retina without choroidal mass or retinal detachment. Anterior segment optical coherence tomography showed thickening of the iris with deposits surrounding the tube shunt (Figure 2A, white arrows). Ultrasound biomicroscopy disclosed a 3-mm thick, dome-shaped echolucent mass in the ciliary body stroma extending from 12 o’clock to 4 o’clock with no extrascleral extension (Figure 2B). The mass measured 20 mm in length and involved only the ciliary body.
The differential diagnosis for the ciliary body mass included neoplasms of the ciliary body stroma, most likely malignant melanoma. Other rarer entities included schwannoma, leiomyoma, lymphoma, metastasis and inflammatory disorders such as juvenile xanthogranuloma and Langerhans histiocytosis. Fine needle aspiration biopsy of the anterior chamber debris and ciliary body mass disclosed discohesive epithelioid cells with scant pigment and high nuclear-cytoplasmic ratio and prominent nucleoli, suspicious for melanoma. The cells were immunoreactive for melanocytic markers Melan-A and HMB 45. The cells also co-expressed cytokeratin marker AE1/AE3, but the intrastromal location of the tumor excluded a ciliary body epithelial tumor. Uveal melanoma seemed most likely, based on the tumor’s clinical, cytopathologic and immunohistochemical features.
Management options included plaque radiotherapy or enucleation. Plaque radiotherapy wasn’t a reasonable option due to the extensive tumor size with total aqueous seeding and the presence of a tube shunt with possible extraocular tumor seeding, so the left eye was managed with enucleation. Intraoperatively, care was taken to avoid violating the capsule of the tube shunt and prevention of tumor seeding, so the globe and tube shunt were removed together as one piece. Gross pathology showed a ciliary body mass that measured 20 mm in circumferential base, 8 mm in anteroposterior base and 2.5 mm in thickness (Figure 3A).
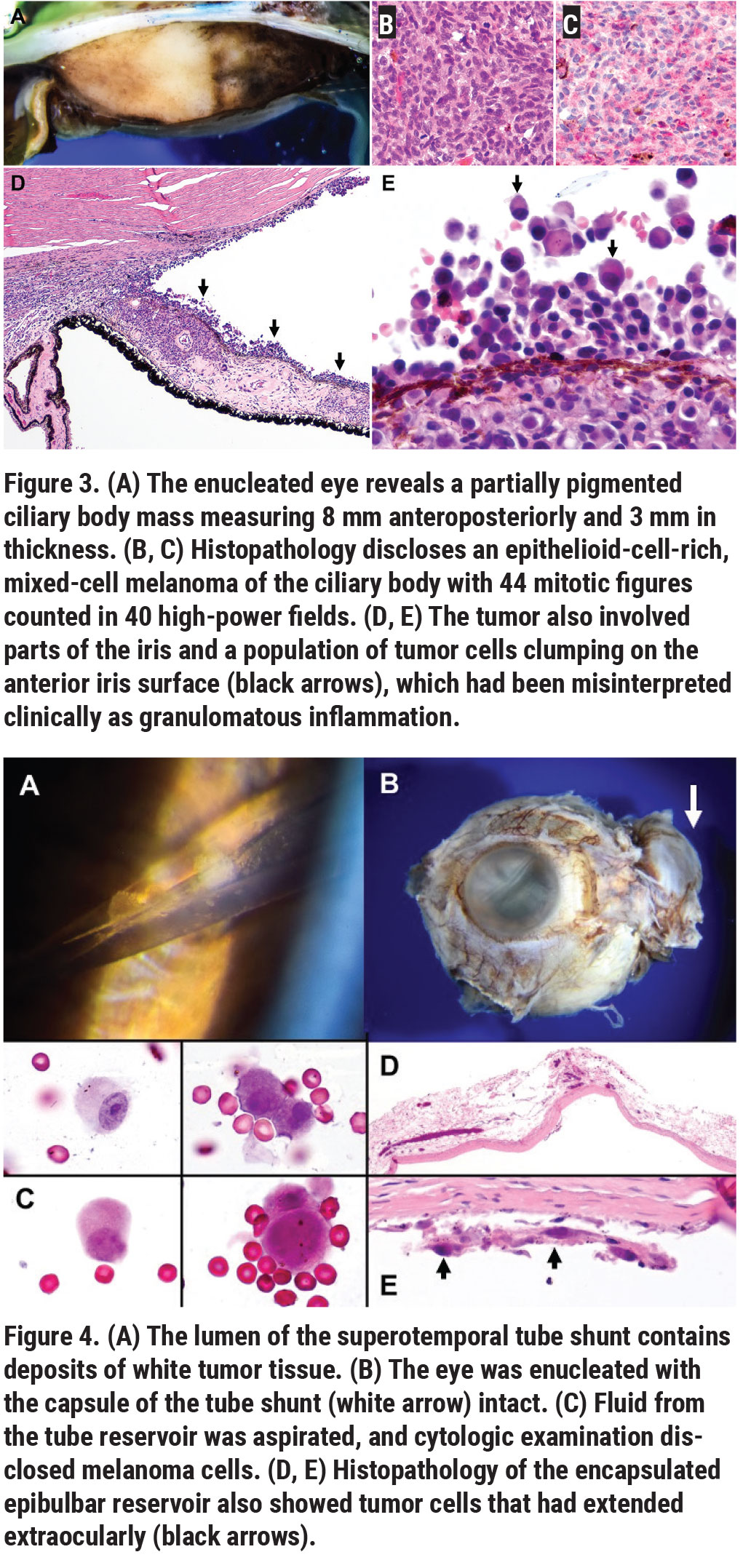 |
Histopathology disclosed epithelioid-cell-rich mixed-cell melanoma with mitotically active “hotspots” containing two to three mitoses per high-power field (Figure 3B). The tumor cells were mitotically active, and their nuclei failed to stain for BRCA1-associated protein 1 (BAP1), consistent with a BAP1 mutation and a significant risk for metastatic disease (Figure 3C). The tumor involved the peripheral iris and had spawned a population of discohesive amelanotic epithelioid cells that seeded the anterior segment, blanketing the iris and angle and forming the deposits and clumps of cells that were initially misinterpreted clinically as granulomatous inflammation (Figure 3D, black arrows, Figure 3E).
Clinical examination of the tube shunt revealed tumor cells within the shunt (Figure 4A), and that the globe with the shunt capsule (white arrow) was intact following enucleation (Figure 4B). The fluid contents of the shunt disclosed melanoma cells that had extended into the drainage device (Figure 4C). The shunt capsule revealed rare tumor cells, some of which had extended extraocularly (Figure 4D, Figure 4E, black arrows). Additional orbital biopsies were tumor-free. The final diagnosis was mitotically active ciliary body “ring” melanoma with tumor seeding into the anterior segment and extension into the Ahmed valve shunt.
The patient healed well, and a prosthesis was placed. She was referred to a melanoma oncologist for consideration of adjuvant Sunitinib, a receptor protein-tyrosine kinase inhibitor, to help prevent metastatic disease. The results of her tumor genetic testing showed chromosome 3 monosomy and multiple 8q amplifications consistent with The Cancer Genome Atlas (TCGA) Group D, implying a high risk for metastasis. Blood test for germline BAP1 mutation is pending. Going forward, she’ll require regular surveillance with magnetic resonance imaging of the orbit, liver function tests, liver MRI and chest X-rays to monitor for metastatic disease.
Discussion
Ciliary body melanoma is rare, comprising 6 percent of all uveal melanomas, with uveal melanomas occurring at an incidence of 5.1 cases per million per year.1 In addition, CB melanomas infrequently occur in people under the age of 30, as in this patient’s case.2 Diagnosis of CB melanoma is challenging, since the tumors are often hidden behind the iris without retinal detachment, until they become large and possibly sublux the lens.2,3 Consequently, the tumors are often advanced by the time they’re diagnosed, with a mean basal thickness of 6.6 mm at diagnosis.1 Ultrasound is a useful tool for identifying ciliary body melanoma.3,4
It’s not unprecedented for an intraocular tumor to cause increased intraocular pressure and glaucoma.5 In a study of over 2,500 eyes with intraocular tumors, 5 percent were found to have secondary glaucoma.6 It’s estimated that 17 percent of CB melanomas result in secondary glaucoma.6 This can occur by a variety of mechanisms, including direct tumor invasion of the iridocorneal angle, inflammation from tumor necrosis, pupillary block, tumor seeding into the angle and iris neovascularization.5-8 One study analzyed five glaucomatous eyes that had undergone implantation of tube shunts and were subsequently found to have undiagnosed intraocular tumors: Two had medulloepithelioma and three had uveal melanoma.5 The eyes were misdiagnosed with various types of glaucoma for a mean timespan of more than five years prior to the discovery of tumor on histopathology. Four of the five enucleated eyes had extrascleral tumor extension, and three had extraocular tumor cells in the tube shunt reservoirs.5 The authors concluded that tube shunt implants can provide a pathway for extraocular tumor spread, and they speculated that the difficulty in visualizing a ciliary body tumor was a crucial factor in the failure to recognize tumor-induced glaucoma.5
Less commonly, ciliary body melanoma can masquerade as sclerouveitis, which is thought to be caused by tumor necrosis inducing inflammation, and the presence of sentinel episcleral vessels, simulating scleritis.9,10 In one case series, two patients were misdiagnosed with scleritis from misinterpretation of sentinel episcleral vessels feeding the tumor and inflammation, which transiently improved with oral and topical corticosteroids.11 Others have observed patients with uveitis, including anterior chamber inflammation, yellow nodules on the iris and injected eyes, who in fact had occult uveal melanoma.9,10,11 In many of these cases, imaging was an important diagnostic tool for identifying tumors in eyes misdiagnosed as having chronic uveitis. In particular, UBM offers a fairly high-resolution tool for visualizing the ciliary body at all clock hours for detection of tumors that might not be seen on examination.
Our patient was unique not only in her presentation, but also in her young age (27). Testing for BAP1 syndrome is an important part of the workup for patients who are young or have a family history of multiple cancers, including melanoma.12 BAP1 is a tumor-suppressor gene located on chromosome 3p21.1. When BAP1 is mutated, patients are predisposed to a variety of cancers including uveal melanoma, malignant mesothelioma, renal cell carcinoma, cutaneous melanomas and basal cell carcinoma.2,12 In a prior review of approximately 500 patients with uveal melanoma, 5 percent had BAP1 polymorphisms, which were associated with larger tumors and an increased incidence of ciliary body involvement.2,13
In addition to testing for germline BAP1 mutations, assessing for somatic BAP1 mutation in the tumor helps to stratify the risk for tumor metastasis. Researchers found that somatic BAP1 mutation within the intraocular melanoma was significantly associated with an increased risk for systemic metastasis.14,15 On histochemical immunostaining of this patient’s tumor, there was evidence of BAP1 somatic mutation, suggesting substantial risk for metastatic disease. The molecular cascade involving BAP1 appears to be linked to tumor metastasis and may be a target for treatment in the future.15 τ
Support provided in part by the Eye Tumor Research Foundation, Philadelphia (CLS). The funders had no role in the design and conduct of this study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript. Carol L. Shields, MD has had full access to all the data in the study and takes responsibility for the integrity of the data.
Inquiries: Carol L. Shields, MD, Ocular Oncology Service, 840 Walnut Street, Suite 1440, Philadelphia, PA 19107. Tel: (215) 928-3105, Email: carolshields@gmail.com.
1. Shields CL, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol 2009;127:989–998.
2. Kaliki S, Shields CL. Uveal melanoma: Relatively rare but deadly cancer. Eye 2017;31:241–257.
3. Simpson ER. Ciliary body melanoma: A special challenge. Can J Ophthalmol 2004;39:365–371.
4. Kawana K, Okamoto F, Nose H, Oshika T. Ultrasound biomicroscopic findings of ciliary body malignant melanoma. Jpn J Ophthalmol 2004;48:412–414.
5. Kaliki S, Eagle RC, Grossniklaus HE, et al. Inadvertent implantation of aqueous tube shunts in glaucomatous eyes with unrecognized intraocular neoplasms: Report of 5 cases. JAMA Ophthalmol 2013;131:925–928.
6. Shields CL, Shields JA, Shields MB, Augsburger JJ. Prevalence and mechanisms of secondary intraocular pressure elevation in eyes with intraocular tumors. Ophthalmology 1987;94:839–846.
7. Camp DA, Yadav P, Dalvin LA, Shields CL. Glaucoma secondary to intraocular tumors: Mechanisms and management. Curr Opin Ophthalmol 2019;30:71–81.
8. Stadigh A, Puska P, Vesti E, et al. Ring melanoma of the anterior chamber angle as a mimicker of pigmentary glaucoma. Surv Ophthalmol 2017;62:670–676.
9. Al-Haddab S, Hidayat A, Tabbara KF. Ciliary body melanoma with optic nerve invasion. Br J Ophthalmol 1990;74:123–124.
10. Nguyen QD, Foster CS. Ciliary body melanoma masquerading as chronic uveitis. Ocul Immunol Inflamm 1998;6:253.
11. Yap EY, Robertson DM, Buettner H. Scleritis as an initial manifestation of choroidal malignant melanoma. Ophthalmology 1992;99:1693–1697.
12. Masoomian B, Shields JA, Shields CL. Overview of BAP1 cancer predisposition syndrome and the relationship to uveal melanoma. J Curr Ophthalmol 2018;30:102–109.
13. Gupta MP, Lane AM, DeAngelis MM, et al. Clinical characteristics of uveal melanoma in patients with germline BAP1 mutations. JAMA Ophthalmol 2015;133:881–887.
14. Ewens KG, Lalonde E, Richards-Yutz J, et al. Comparison of germline versus somatic BAP1 mutations for risk of metastasis in uveal melanoma. BMC Cancer 2018;18:1172.
15. Harbour JW, Onken MD, Roberson EDO, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010;330:1410–1413.



