Presentation
A 5-year-old male presents with incidentally found heterochromia. Approximately one year prior to presentation, the patient’s mother noted that the child’s left eye was darker than his right eye. The patient himself did not report any symptoms or visual changes. An optometrist’s examination at the time was reportedly normal. One year later, the patient was evaluated by an ophthalmologist who noted vitreous hemorrhage in the left eye with presence of an intraocular mass. The patient was then referred for evaluation by the Wills Ocular Oncology Service.
History
The patient was born at full term without complications, and didn’t have any notable past medical or ocular history. He didn’t take any medications. Family history didn’t reveal any cases of cancers or early childhood blindness. Review of systems was negative.
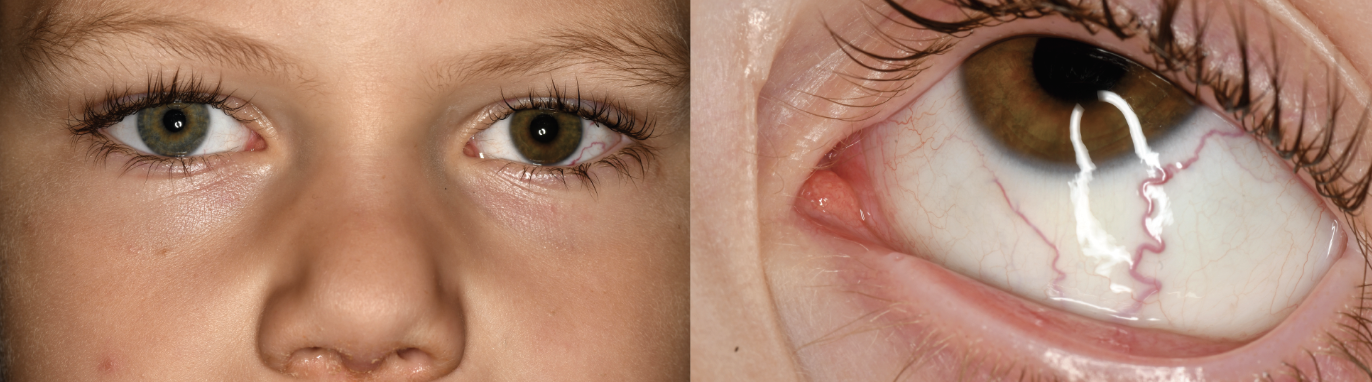 |
| Figure 1. External photographs showing green colored iris of the right eye and brown colored iris of the left eye with an inferotemporal episceral sentinel vessel. |
Examination
Ocular examination demonstrated visual acuity of 20/25 in the right eye and light perception in the left eye. Pupils were reactive with no evidence of an afferent pupillary defect. Extraocular movements were full bilaterally. Intraocular pressures were 13 mmHg
bilaterally.
Anterior segment evaluation revealed heterochromia with green-colored iris in the right eye and brown-colored iris in the left eye (Figure 1). There was no iris neovascularization. In the left eye, there was a dilated, tortuous sentinel episcleral vessel overlying the inferotemporal quadrant of the left sclera (Figure 1). The left eye had a dense cataract obscuring the view to the fundus.
Dilated fundus examination revealed normal findings in the right eye, including clear vitreous without hemorrhage or cells (Figure 2). The left vitreous was noted to have pigment dispersion in the anterior vitreous with hazy view to the optic nerve, macula and vessels (Figure 2). There was a large intraocular mass appearing to arise from the inferotemporal ciliary body measuring 16 by 16 mm.
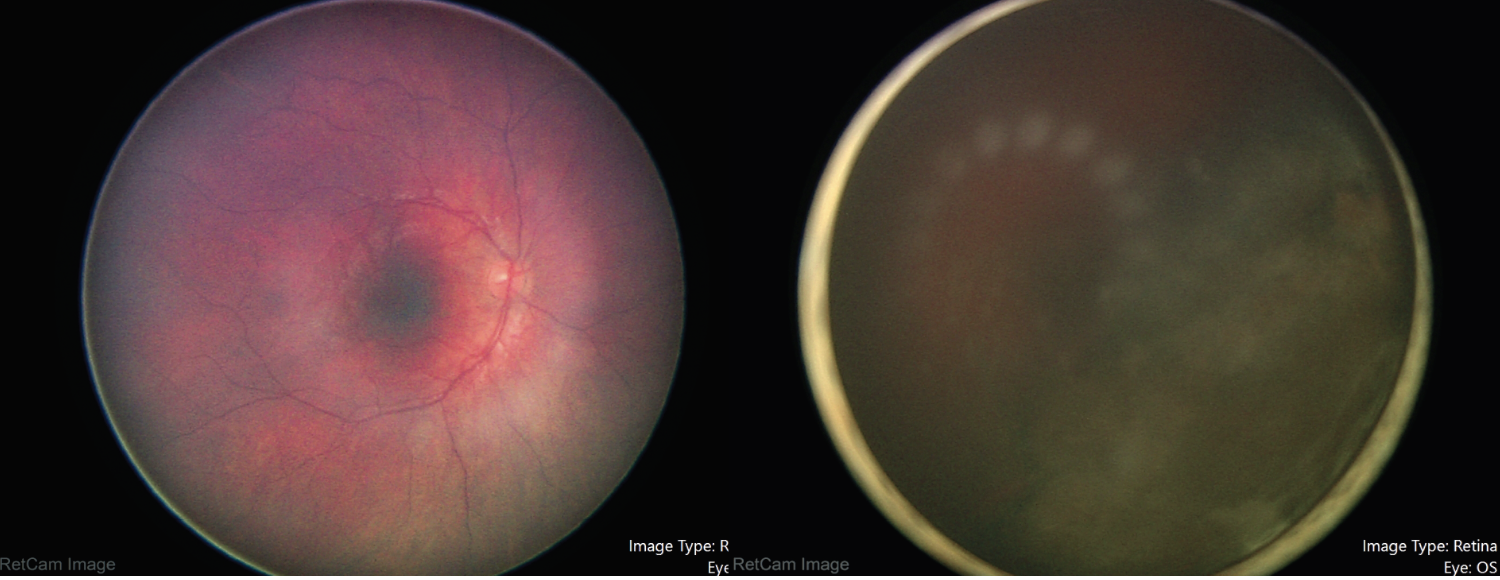 |
| Figure 2. Fundus photography of the right and left eye during examination under anesthesia, showing normal findings in the right and hazy view with an inferotemporal intraocular mass in the left eye. |
What’s your diagnosis? What further work-up would you pursue? The diagnosis appears below.
 |
| Figure 3. Transscleral illumination highlighting an inferotemporal mass extending from 2 to 6 o’clock in the left eye. |
Work-up, Diagnosis and Treatment
Our suspicion was for ciliochoroidal melanoma, but since the patient was only 5 years of age, we performed fine needle aspiration biopsy to differentiate melanoma from melanocytoma, pigmented medulloepithelioma or adenoma. The patient was taken to the operating room for an examination under anesthesia. Scleral transillumination revealed an anteriorly located tumor extending from 2 to 6 o’clock (Figure 3). A/B-scan ultrasound showed a ciliochoroidal mass with a thickness of 11.8 mm and a base of 13.5 mm (Figure 4). Ultrasound biomicroscopy showed the mass originated within the ciliary body, with the tumor pushing on the lens causing cataract (Figure 4). There was no evidence of extraocular extension. Fluorescein angiography demonstrated a hyperfluorescent tumor posterior to the lens with no view of the retinal vessels (Figure 5).
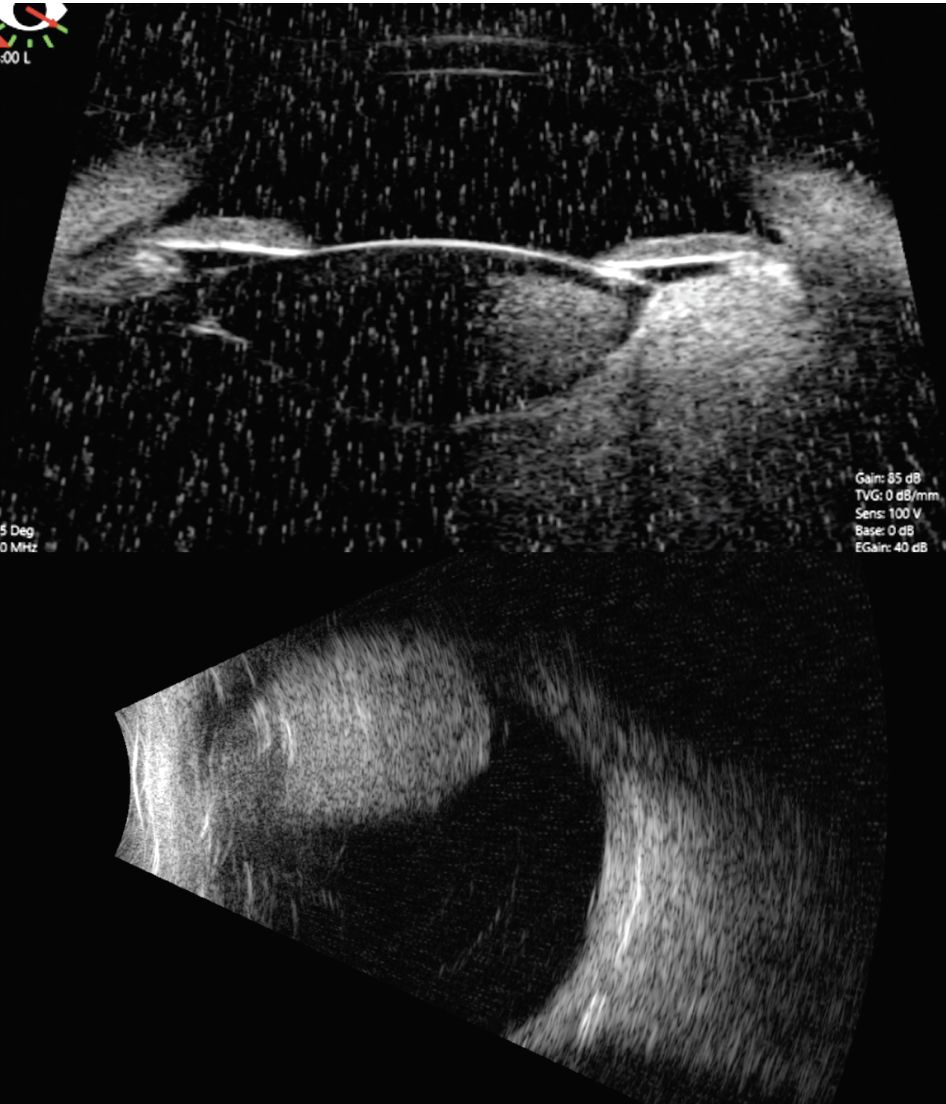 |
| Figure 4. A/B-scan ultrasonography and ultrasound biomicroscopy showing the mass arises from the ciliary body and measures 11.8 mm in thickness and 13.5 mm at its base. |
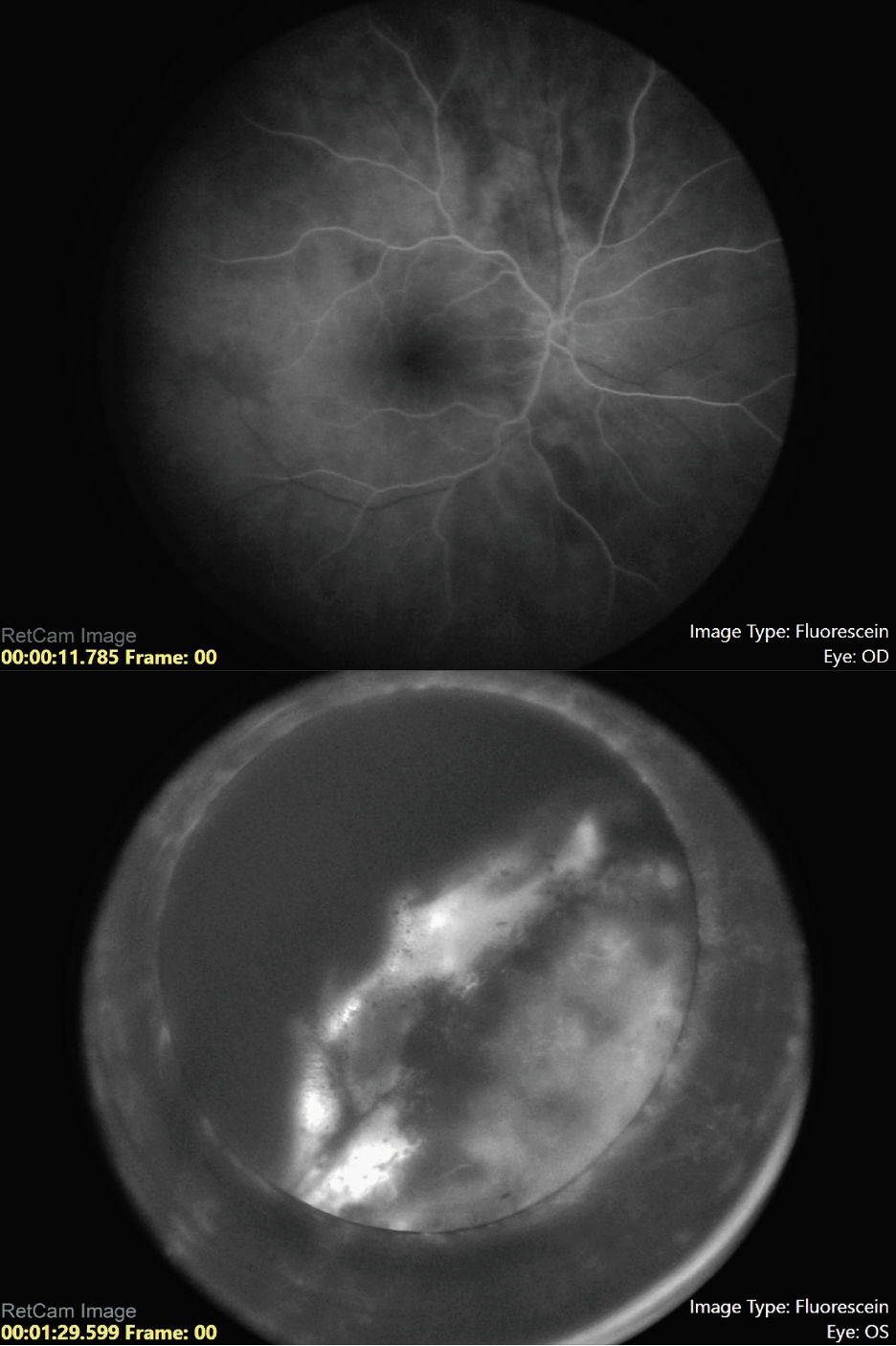 |
| Figure 5. Fluorescein angiography of the right and left eye, respectively, showing a hyperfluorescent tumor posterior to the lens in the left eye with no view of the retinal vessels. |
The patient underwent fine needle aspiration biopsy of the mass, which revealed epithelioid cell type malignant melanoma. Given the pathologic diagnosis and extent of the malignant tumor, a decision was made to proceed with enucleation. Gross pathology of the globe is shown in Figure 6. Microscopic examination was notable for dyscohesive cells with loosened intercellular connections, a feature of malignancy (Figure 7). Interestingly, the specimen contained many binucleate and trinucleate cells with relatively few mitotic figures (Figure 8).
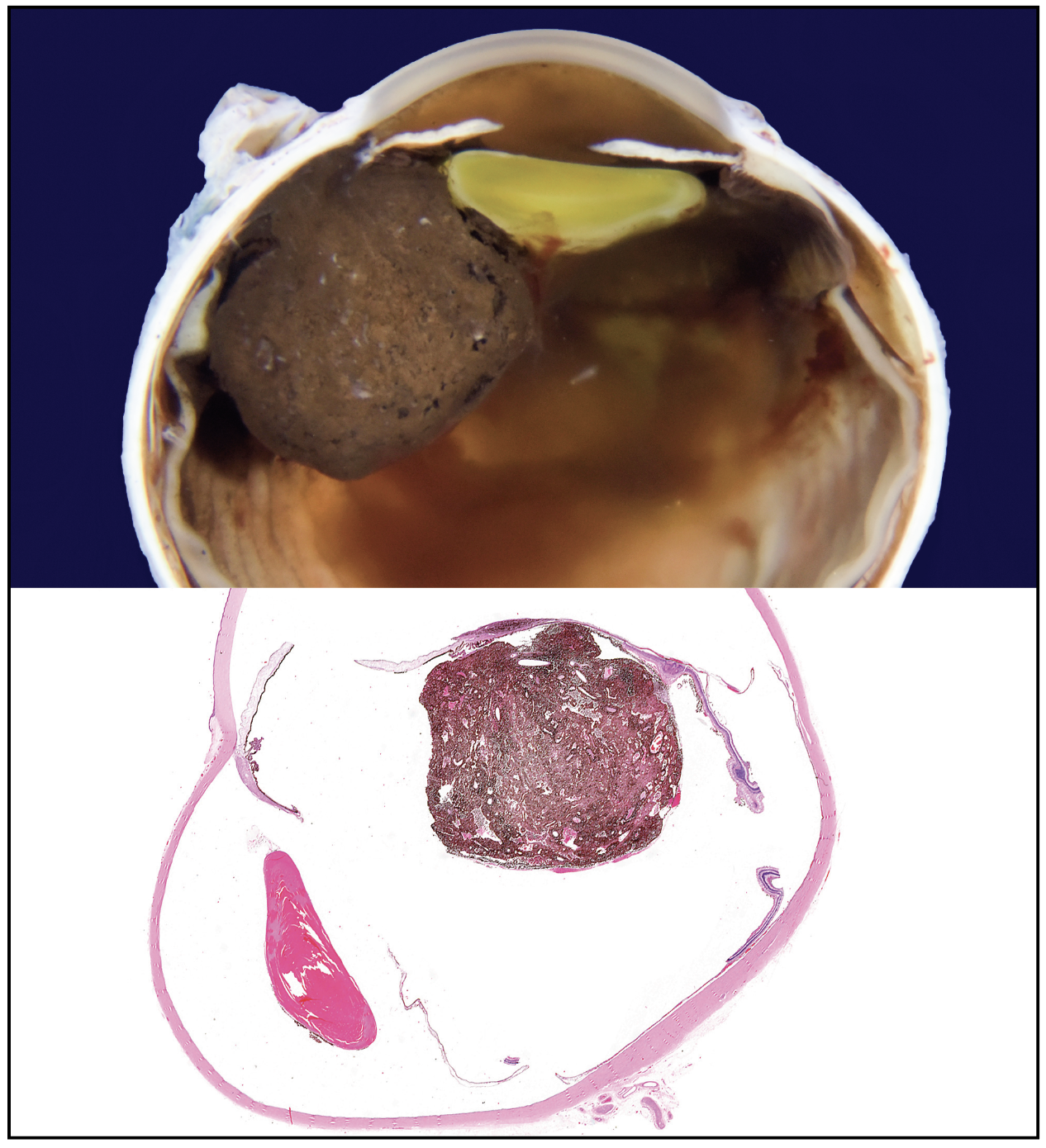 |
| Figure 6. Gross pathology of the enucleated eye showing a tumor based in the ciliary body stroma and mushrooming into the ocular cavity. |
Discussion
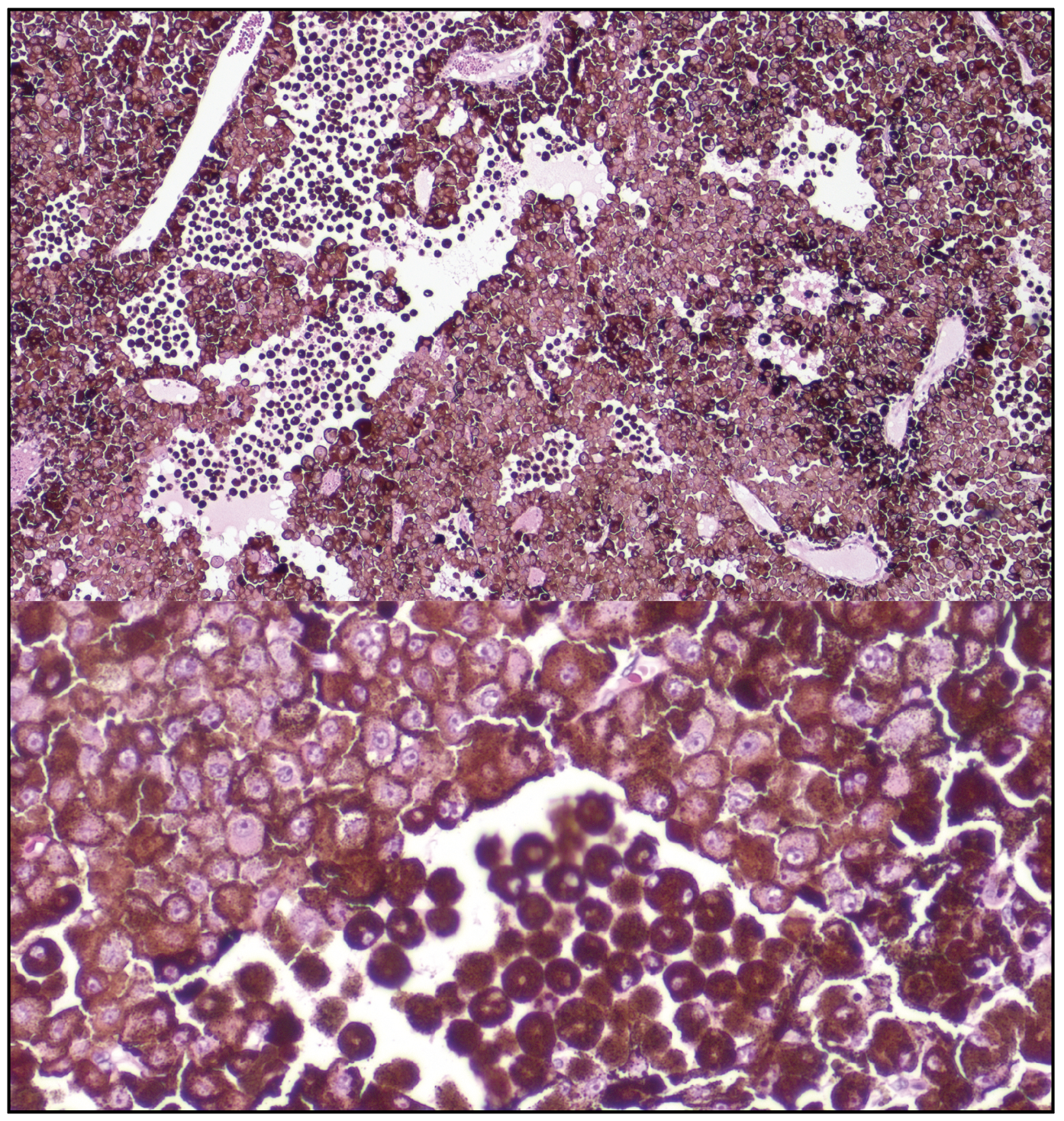 |
| Figure 7. (Top) Microscopic examination of the tumor cells show dyscohesive epithelioid cells with loosened intercellular connections, a feature of malignancy. (Bottom) A zoomed view of these tumor cells, adjacent to a population of melanophages. |
Our patient is a 5-year-old male who first presented with asymptomatic acquired heterochromia. The differential diagnosis for acquired heterochromia includes sympathetic-innervation heterochromia, ocular trauma, Fuchs’ heterochromic uveitis, iris neovascularization and pigment dispersion on the iris whether from nevus or melanoma. In sympathetic-innervation heterochromia such as Horner syndrome or neuroblastoma, unilateral lack of sympathetic innervation interferes with melanin production in melanocytes. In contrast, cases of melanocyte infiltration such as diffuse nevus or melanoma cause increased iris pigmentation in the affected eye.1 In this case, the acquired heterochromia was related to congenital uveal melanocytosis that gradually darkened in the first few years of life and lead to development of the ciliary body melanoma that was ultimately treated with enucleation.
Uveal melanoma is a rare intraocular tumor that arises from the melanocytes of the iris, ciliary body or choroid.2 It’s the most common primary intraocular malignancy in adults, with a prevalence of 5.1 per million.3 The majority are located posterior to the equator. Ciliary body location is the least common site for uveal melanoma but portends the least favorable prognosis. Tumors with epithelioid cells also have poorer prognosis compared to spindle or mixed spindle-epithelioid cell types.4-7
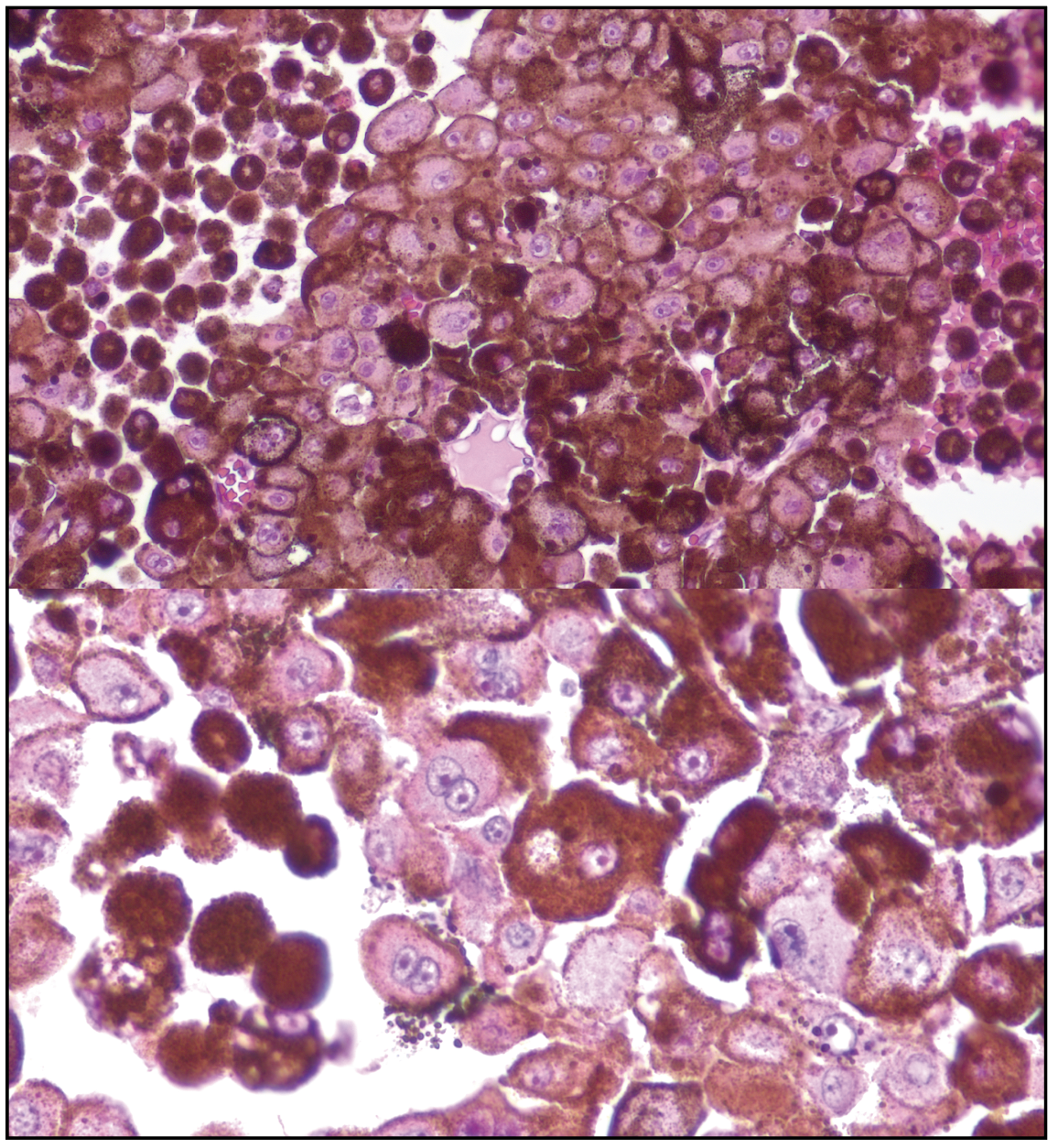 |
| Figure 8. Close examination of the epithelioid cells shows low mitotic figures and prominent pleomorphism, with many binucleate and trinucleate tumor cells. |
The biggest advance in prognostication has been through understanding the molecular mechanisms driving this malignancy. In a large retrospective cohort study of 1,001 eyes with uveal melanoma, cases were categorized according to The Cancer Genome Atlas based on tumor DNA and followed for melanoma-related metastasis at five and 10 years. The Kaplan-Meier rate of liver metastasis, lung metastasis or any distant metastasis was highest in class D tumors with monosomy 3 and gain of chromosome 8q. Rates of metastasis were lowest in class-A tumors with disomy 3 and disomy 8. This classification strategy is used to guide treatment, particularly for class-C and -D tumors that may require adjuvant therapy.8
Compared to that of the adult general population, uveal melanoma in pediatric patients is much rarer and with more favorable prognosis. Based on a large series of 8,033 patients with uveal melanoma, only 1 percent occurred in patients aged 20 years or younger, whereas 53 percent were diagnosed in patients aged 21 to 60 years old and 45 percent diagnosed in patients over the age of 60. Patients under 20 years old with uveal melanoma were more likely to be non-white with lower rates of tumor-related metastasis and death compared to adults, similar to cutaneous melanoma. These patients were more likely to have melanoma located in the iris, more remote from the fovea and optic disc, and with smaller tumor diameter and thickness. It’s speculated that younger patients have a more favorable prognosis independently of tumor size due to declining host defense mechanisms with advancing age.9
Focusing specifically on pediatric choroidal and ciliary body melanomas, a 2016 survey by the European Ophthalmic Oncology Group studied 299 patients, of whom 114 were children younger than 18 years of age and 185 were young adults between the ages of 18 and 25 years. Patients with iris melanoma were excluded from the study. The authors found that adjusting for TNM stage and gender, children with choroidal and ciliary body melanoma have more favorable survival than young adults. Male children tended to have a more favorable survival compared to female children. Similar to the general adult population, higher TNM staging and monosomy 3 with 8q gain predicted the highest risk for metastasis.10
In conclusion, our patient presented with asymptomatic acquired heterochromia that was found to be secondary to a case of pediatric ciliary body melanoma. Cases of pediatric uveal melanoma are rare and require special consideration. Compared to adult uveal melanomas, pediatric cases tend to carry a more favorable prognosis. They share many of the same molecular and genetic basis as their adult counterparts, with similar metastatic outcomes based on The Cancer Genome Atlas categorization. The cytogenetic analysis of tumor DNA and genomic testing for conditions, including BAP1 tumor predisposition syndrome, is still pending to complete the work-up for this unique case.
1. National Center for Biotechnology Information. Heterochromia Iridis - StatPearls - NCBI Bookshelf. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. https://www.ncbi.nlm.nih.gov/books/NBK574499/. Accessed June 8, 2023.
2. Ho A, Brown G, McNamara J, Recchia F, Regillo C, Vander J. Retina: Color Atlas of Synopsis of Clinical Ophthalmology. 2nd Ed. Philadelphia: Lippincott Wiliams & Wilkins, 2012.
3. Singh AD, Turell ME, Topham AK. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011;118:9:1881-5.
4. Singh AD, Kalyani P, Topham A. Estimating the risk of malignant transformation of a choroidal nevus. Ophthalmology 2005;12:10:50-54.
5. Shields CL, Dalvin LA, Ancona-Lezama D, Yu MD, Di Nicola M, Williams BK Jr, Lucio-Alvarez JA, Ang SM, Maloney S, Welch RJ, Shields JA. Choroidal nevus imaging features in 3,806 cases and risk factors for transformation into melanoma in 2,355 cases: The 2020 Taylor R. Smith and Victor T. Curtin Lecture. Retina 2019;26:10:28-36.
6. Shields CL, Kaliki S, Shah SU, Luo W, Furuta M, Shields JA. Iris melanoma: features and prognosis in 317 children and adults. JAAPOS 2012;19:2:23-29.
7. Shields JA, Sanborn GE, Augsburger JJ. The differential diagnosis of malignant melanoma of the iris. A clinical study of 200 patients. Ophthalmology 1983;90:6:716-20.
8. Shields CL, Mayro EL, Bas Z, Dockery PW, Yaghy A, Lally SE, Ganguly A, Shields JA. Ten-year outcomes of uveal melanoma based on The Cancer Genome Atlas (TCGA) classification in 1001 cases. Indian J Ophthalmol 2021;28:7:22-30.
9. Shields CL, Kaliki S, Furuta M, Mashayekhi A, Shields JA. Clinical spectrum and prognosis of uveal melanoma based on age at presentation in 8,033 cases. Retina 2013;20:7:40-45.
10. Al-Jamal RT, Cassoux N, Desjardins L, Damato B, Konstantinidis L, Coupland SE, Heimann H, Petrovic A, Zografos L, Schalenbourg A, Velazquez-Martin JP, Krema H, Bogdali A, Markiewicz A, Romanowska-Dixon B, Metz CH, Biewald E, Bornfeld N, Kiratli H, Bronkhorst IH, Jager MJ, Marinkovic M, Fili M, Seregard S, Frenkel S, Pe’er J, Salvi SM, Rennie IG, Rospond-Kubiak I, Kociecki J, Kiilgaard JF, Heegaard S, Cohen VM, Sagoo MS, Amiryan A, Saakyan S, Eide N, Krohn J, Midena E, Parrozzani R, Grange JD, Kilic E, Blasi MA, Saornil MA, Kivelä TT. The pediatric choroidal and ciliary body melanoma study: A survey by the European Ophthalmic Oncology Group. Ophthalmology 2016;23:4:45-52.



