Last year, European regulatory agencies pulled several models of angle-supported phakic intraocular lenses from the market in response to reports of unreasonable amounts of endothelial cell loss. This move gave surgeons everywhere pause, and caused them to take a hard look at the phakic lenses they were using. To help inform you about the safety profile of the phakic lenses we currently use in the
Europe Pulls AS-IOLs
Corneal endothelial cell density is one of the issues that the U.S. Food and Drug Administration watches carefully with these lenses, and has been a problem with some devices, such as the phakic anterior chamber angle-supported IOLs.
The concerns began after the release of a 2006 Ophthalmology article that retrospectively analyzed 100 eyes of 66 angle-supported phakic IOL patients who had to have their lenses explanted at the practice of the article's first author, Jorge Alio, of
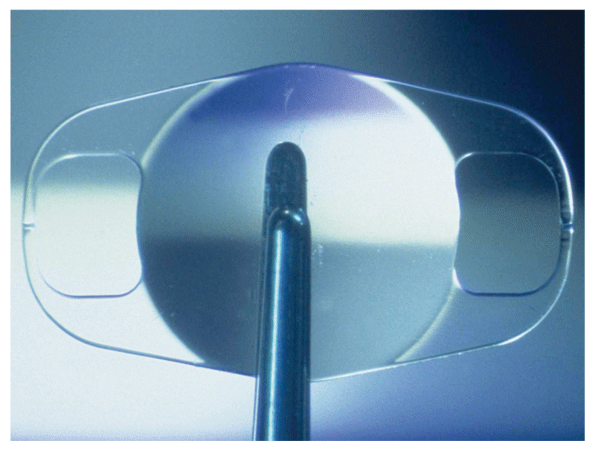
Phakic lenses that are approved in the United States, such as the AMO Verisyse (above), have shown a trend toward stable endothelial cell counts.
In response to this, the European regulatory authorities, with
Current and Future Lenses
In all of the clinical trials for the phakic lenses currently available in the
The Staar Visian sits in the posterior chamber, and, if the location of the implant in relation to the cornea is related to mechanical cell loss, one might presume the Visian's location helps avoid endothelial damage.
After three years of follow-up, cumulative cell loss with the Visian is just under 9 percent, and seems to be hitting a plateau at 12.5 percent between four and five years. So, there does appear to be cell loss associated with the actual surgical implantation, but whether or not this loss progresses is a question we can't definitively answer yet. However, we remain optimistic about the endothelial health post-Visian implantation, and will continue to monitor it going forward.
The iris-fixated Verisyse lens sits in an intermediate position between the angle and the posterior chamber. It's not in direct apposition to the angle or the peripheral cornea, therefore, simply based on location alone, it presumably may have a risk profile between that of an angle-supported lens and a posterior-chamber IOL.
Based on data from
Overall, though it appears there's a trend toward stabilization in endothelial cell density over time with these two lenses, the numbers currently aren't large enough to prove anything at a statistically significant level. Therefore, we'll continue to watch these cells carefully. Many of us feel these lenses remain a reasonable alternative for certain patients. In fact, they continue to show increased use, demonstrating surgeons' confidence. 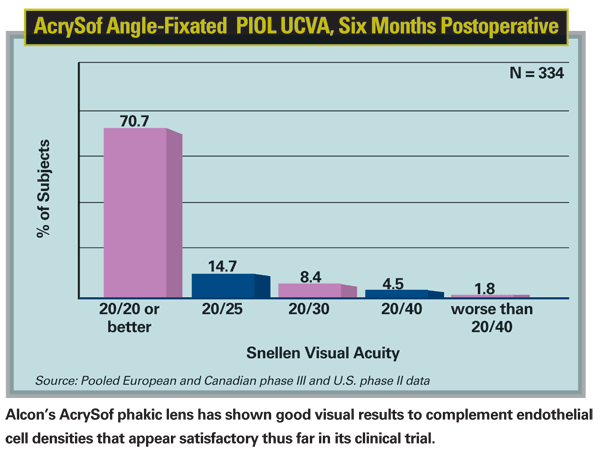
Finally, there is yet another version of angle-supported lens in the pipeline, the Alcon AcrySof anterior chamber angle-supported phakic lens, of which I am an investigator in the
The cell densities with the AcrySof lens have been quite good. The data reported in both the two- and three-year studies from Europe and early results from the
What all of this means, in light of the trend toward implanting more of these lenses, is that continuous monitoring of endothelial cell density is now a must. We'll remain cautiously optimistic about the future of these IOLs, and will pass on what we learn about them to patients so they can make the most informed decision about their potential surgeries.
Dr. Vukich is director of the
1. Alió JL, Abdelrahman AM, Javaloy J, et al. Angle-supported anterior chamber phakic intraocular lens explantation causes and outcome. Ophthalmology 2006;113:12:2213-20.
2. Javaloy J, Alió JL, Iradier MT, et al. Outcomes of ZB5M angle-supported anterior chamber phakic intraocular lenses at 12 years. J Refract Surg 2007;23:2:147-58.