Injecting fluid into the eye raises the intraocular pressure, which can go as high as 90 mmHg when a typical volume of anti-VEGF medication is injected. In a healthy eye, any pressure rise that occurs is short-lived. However, the intraocular pressure in some patients remains chronically high after an injection. A glaucomatous eye might have trouble managing the increased pressure caused by the injection, but many patients who have experienced this sustained pressure increase had not previously been diagnosed with glaucoma. As a result, the reason for their sustained pressure increase has been something of a mystery.
Here, I’d like to share some of my experience with these patients and some of the work my colleagues and I have done to try to elucidate what might be causing this pressure rise following anti-VEGF injections.
Case Histories
A few years ago I saw a 68-year-old Caucasian male patient who had been diagnosed with wet age-related macular degeneration in his right eye. He was given an injection of 1.25 mg of Avastin the day of his AMD diagnosis—the first such injection he’d ever received. He returned to the retina specialist two weeks later complaining of pain in that eye and blurred vision; his IOP in that eye was 56 mmHg. There was no vitreous or anterior chamber inflammation detected on the dilated exam, so it was apparently not a reaction to the drug. The patient was referred to me for further analysis.
The patient had no history of glaucoma in that eye, no family history of glaucoma, no prior surgery or trauma and no history of steroid use that might account for the high pressure. Slit lamp exam revealed slight corneal edema, but there was no sign of inflammation and no signs suggesting a secondary form of glaucoma such as pigment dispersion or pseudoexfoliation. In my exam, the pressure by Goldmann applanation was 53 mmHg and the angle was clear and open. The rest of the exam was essentially unremarkable. The possibility that this was injection-related was obvious, but the explanation for the lingering elevated pres-sure was not. This patient ended up getting an Ahmed valve several weeks later, and his wet macular degeneration treatment was changed, first to Lucentis and then to Eylea. The patient is doing well.
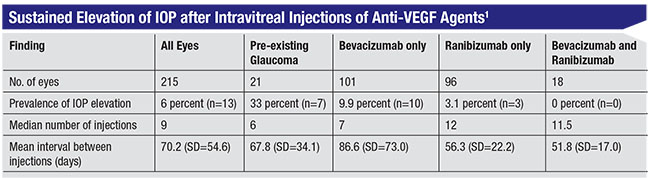
This experience inspired me to look further into this mystery, and I decided to conduct a chart review. A medical student, who became the first author on the paper we eventually published,1 spent time in my clinic, as well as in the clinic of a referring retina specialist in our community. Looking at the charts of 215 patients who had received consecutive injections, what we found was remarkable. Thirty-three percent of patients with pre-existing glaucoma had a significant elevation of pressure after anti-VEGF injections that was sustained over time, compared to 3 percent of patients without pre-existing glaucoma—despite a lower mean number of injections (p<0.001). And although we did see some pressure elevation following Lucentis injections, the rate was about three times higher in the Avastin group.
When the paper was published, I started getting phone calls from people around the country who were noticing this phenomenon. In 2009, John Carver, MD, reported a series of his own patients who were being treated monthly with Avastin for choroidal neovascularization, with two-year follow-up. Twelve percent of those eyes developed an unexpected IOP rise, usually to between 30 and 40 mmHg, that showed “remarkable resistance” to multiple topical drug therapies.
We decided to chart the locations of the cases we were hearing about. The main thing we noticed was that these cases were happening in clusters; the problem wasn’t widespread, and in some cases it was present in some practices but not in others in the same city. The latter practices wondered why we were asking about it; they’d never seen it. This didn’t help us draw any conclusions about the cause or mechanism that might be involved, but it did raise one possible association: Different practices in the same area often use different compounding pharmacies.
Digging Deeper
We wondered whether we could pin down the root cause of this, so we began systematically testing different hypotheses in the lab. Our first question was: Could this possibly be a toxicity issue? Although Lucentis was tested in the eye before approval by the U.S. Food and Drug Administration, Avastin was not.
We exposed trabecular meshwork cells to different levels of Avastin in vitro. The initial tests showed that Avastin could indeed be toxic to trabecular meshwork cells, but only at concentrations that were much higher than what we inject into the eye. Notably, we did not see this effect with Lucentis at high-dose molar equivalents. But given that we were only seeing toxicity with very high concentrations of Avastin, that didn’t seem to be a likely explanation for this phenomenon.
A second hypothesis we pursued was the possibility that the concentration of Avastin in the injections was somehow being altered; that could mean that some patients were receiving more Avastin than the physician realized, potentially leading to toxicity. To test this hypothesis, we started buying vials of Avastin straight from our Genentech distributor, as well as compounded Avastin (in syringes) from different pharmacies around the country, hoping to see whether something about the compounding process was raising the concentration of Avastin.
What we found was the opposite. The level of the Avastin protein in the compounded samples was lower than the level in the drug coming straight out of the vial. This meant there was a loss of protein during the compounding, storage and/or shipping. The cause wasn’t clear—proteins could theoretically be altered by temperature, storage conditions, shipping trauma and other factors—but a lower concentration meant less likelihood of toxicity, not more.
Our third hypothesis was that the elevated pressure was caused by contamination; perhaps there was something else in the vial that we weren’t accounting for. We per-formed a number of experiments, trying to do as many analyses as possible. We didn’t find much, until we did microflow imaging—very fine, focused imaging of the fluid samples.
The difference between them was startling. The fluid taken straight out of the vial was basically clear, except for one tiny shard of glass near the bottom. (That’s pretty typical at this level of imaging. It’s a well-known phenomenon, and it’s harmless.) In contrast, the samples from some of the compounding pharmacies were full of what looked like bubbles. We eventually determined that these were microscopic bits of silicone that were leaching from both the stopper and inside lumen of the syringe. This was not a subtle effect. There were millions of these bubbles, but they were very tiny—less than 10 µm in diameter. That’s too small to be visible to the naked eye, but potentially large enough to block outflow through the trabecular meshwork.
 |
How Did the Particles Get There?
One important question was: How did these microparticles end up being released into the fluid? To answer that question we studied some different repackaging processes to see how they might influence the amount of silicone leaching. We found that several things that could happen to the syringes had this effect:
• Long-term storage. Silicone leaching appears to be somewhat time-dependent. If you put this fluid in a syringe and leave it there for a long time, it increases the number of “bubbles” seen in the sample.
• Going through a freeze-thaw cycle. When mailing the drug, ice blocks are put into the boxes to keep the drug from warming too much. However, the syringes may touch the ice blocks, resulting in the fluid freezing; once you receive it and put it in the refrigerator, the fluid thaws. That freeze-thaw cycle causes millions of bubbles to leach off the plunger and the wall of the syringe.
• Shipping trauma. When a box is shipped, it may be thrown into a truck or onto a loading dock, and it could even be inadvertently knocked around at your practice after arrival. We found that every incidence of mechanical stress increased the amount of leachables seen in the fluid.
Other Explanations
It’s important to note that silicone leaching might not be the only explanation for the chronic IOP rise seen in some patients (although it’s almost certainly a key contributor to the problem in some cases). Other possible explanations include the mechanical impact of repeated in-creases in IOP following injections, which could be taxing the trabecular meshwork. And it’s possible that the anti-VEGF drugs themselves directly impact the trabecular meshwork cells in some patients.
I believe these explanations are less likely to be correct, for a couple of reasons: 1) Many patients (as in the case history mentioned earlier) experience this chronic pressure rise starting immediately after their first injection. 2) No one has demonstrated that anti-VEGF drugs harm the trabecular meshwork. 3) If these explanations were correct, we’d expect to see a more even distribution of the problem. Nevertheless, other explanations for the problem can’t be ruled out, including the possibility that other contaminants are present in the fluid besides the silicone “bubbles.”
The other reason I suspect that silicone leaching is the most likely explanation is that we haven’t seen a single case of chronic elevated pres-sure related to anti-VEGF injections since we started drawing up our own medication straight from the vial, rather than using compounding pharmacies. Other practices in our area have also made the same change, and they report the same drop in the incidence of post-injection chronic pressure rise. In addition, I’m aware that some compounding pharmacies have changed their practices because of our publications, and the number of cases of this appears to be dropping, based on anecdotal reports.
Avastin vs. Lucentis
One question that’s also worth considering is why this problem has been seen in connection with Avastin far more often than Lucentis. There are several differences between the two drugs that might help explain this. For one thing, Lucentis went through the rigorous process of getting FDA approval for use in the eye, including packaging standards. Avastin did not. (For example, Lucentis is drawn from a vial in the clinic; until recently, it was not available in a syringe.) Also, Lucentis was formulated specifically for use in the eye; Avastin was not.
In addition, the drugs themselves are somewhat different. Lucentis is what is called a Fab fragment; it’s a portion of an immunoglobulin molecule, whereas Avastin is the entire molecule. The Avastin mole-cule looks like a letter Y; the leg or stem of the Y is what’s called a fragment crystallizable, or Fc, region. Trabecular meshwork cells have a receptor for Avastin’s Fc region that allows the cells to capture the Avastin molecule and internalize it, which is why Avastin can become toxic in high doses. In contrast, Lucentis doesn’t have an Fc fragment. Some researchers have speculated that this difference might explain why post-injection chronic pressure elevation is seen more often with Avastin than Lucentis, but this is conjecture.
It may be that the few cases of this seen with injections of Lucentis are caused by one or more of the alternate explanations that have been offered. We know that when you inject things into the eye on a regular basis, a certain portion of patients will develop elevated IOP. This has been reported in the literature for a long time. If 1 or 2 percent of Lucentis patients develop chronic high pressure, it’s possible that these patients had a predisposition for glaucoma that was simply not identified. Again, this is all speculation at this point. (I would add that Avastin, Lucentis and Eylea are all routinely used in my institution; I have no preference for one over the others from a safety standpoint, in the setting of proper handling and use.)
Addressing the Problem
What can we do to help ensure that our patients don’t suffer from chronic elevated pressure following anti-VEGF injections? First of all, be vigilant about checking pressure in these patients. Patients who are receiving anti-VEGF injections should have their pressure checked at every visit. (This isn’t always done.) They should be checked before the initial injection and also on subsequent visits prior to any further injections.
Be especially careful if the individual has pre-existing glaucoma. These individuals are especially susceptible to chronic pressure elevation because they already have a dysfunctional outflow system. In this situation:
• Check the patient’s pressure both before and after injection. If a glaucoma patient is noted to have high pressure a half hour after an injection, you can ask that patient to stay a little longer and then check it again.
• If a glaucoma patient has a longer-lasting elevation in IOP after intravitreal injections, be sure to note it in the medical record.
• Consider tapping the anterior chamber before the injection. If a patient has glaucoma, the surgeon can tap the anterior chamber by making a paracentesis to decrease the pressure in the eye before doing the injection. That minimizes the volume effect when you inject the drug.
• Consider pretreating with glaucoma medications. This also decreases the pressure so the volume effect won’t be as massive.
• Consider increasing the glaucoma therapy to help stabilize the elevated pressure. This won’t help in every case, but it should help some patients.
• Follow the patient closely. If the patient continues to have this problem—especially if your efforts to lower the pressure aren't working well—you may want to consider other approaches to addressing the macular degeneration.
• Give it a little time before resorting to surgery. We’ve had patients whose chronic elevated pressure did eventually clear. Unfortunately, that’s not always the case; sometimes the only alternative is to do surgery to relieve the pressure.
Of course, some of these ap-proaches won’t prevent or resolve the problem if flow through the trabecular meshwork is being blocked by tiny silicone particles. But when dealing with a glaucoma patient, these strategies might help to decrease the pressure burden on the eye and limit pressure-related damage to the optic nerve. REVIEW
Dr. Kahook is The Slater Family Endowed Chair in Ophthalmology, professor of ophthalmology, chief of the Glaucoma Service and vice chair of clinical and translational research at the University of Colorado School of Medicine in Aurora, Colo. He is a consultant for and receives research support from Allergan and Alcon.
1. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol 2011;95:8:1111-4.



