Relying on the Zeiss Visumax femtosecond laser, the only laser that can be used for SMILE, I now use the procedure for 40 percent of my refractive surgeries. However, I’d be the first to caution you against performing more SMILE cases for the sake of embracing it as your favored modality. When used properly, SMILE is a safe and effective solution for -1 to -9 D of myopia, with myopic astigmatism up to 3 D. Exceeding this correction range, which some surgeons around the world routinely do, is courting trouble, as is taking an aggressive approach that doesn’t respect the risks of causing ectasia, a torn cap or other unwanted outcomes.
In this article, having used and studied SMILE for five years, I’ll review some newer techniques that can make the procedure easier to perform and some advantages SMILE has over LASIK. I’ll also discuss how to avoid and manage complications and meet my triple objectives of reduced risk, surgical success and predictability.
Promising Start
 |
|
Figure 1. Successfully mastering suction with a femtosecond laser can be a challenge for surgeons learning SMILE, especially if they have no experience creating LASIK flaps. |
When LASIK was introduced across the world in the 1990s, surgeons reported many flap complications, incidents of epithelial ingrowth, flap amputations and complications during and after surgery, including diffuse lamellar keratitis. We’ve learned many lessons from these experiences. That’s largely why we now know how to handle many of the similar complications with SMILE, which was approved for use in the United States in 2016 and has been administered to more than 3 million people in around 70 countries. For example, my search of the literature finds that SMILE has been associated with only 11 to 19 eyes with ectasia—a stark contrast to more than 1,450 eyes that have been linked to ectasia in LASIK.
Besides benefiting from the lessons we’ve learned from LASIK’s longer history, surgeons who successfully provide SMILE do so by thoroughly screening patients and never force-fitting the procedure onto less-than-ideal candidates. As long as you undergo proper training and education, you’ll find SMILE to be safe and predictable in these cases.
Patient Selection
Besides a needed correction of -1 to -9 D of myopia and up to 3 D of myopic astigmatism, patients who qualify for SMILE should have a mesopic pupil size that measures less than 7 mm, a residual stromal bed of greater than 250 µm and a central corneal thickness greater than 475 um. Expected post-procedure keratometry should be between 35 D and 47 D, and all patients should have at least a one-year history of a stable refraction within +/- 0.5 D.3
We recommend SMILE for patients who play contact sports (at risk for dislocating a LASIK flap), who have mild dry eye (at risk for more severe dry eye after LASIK) and larger pupils (because the SMILE lenticule provides a larger optical zone that can potentially reduce the amount of induced higher-order aberrations).
Meanwhile, we steer patients away from SMILE if they have epithelial basement membrane dystrophy, corneal opacity, irregular or scarred corneas, irregular corneal astigmatism and ocular allergies. Other contraindications include corneal thinning disorders (such as keratoconus or central corneal thickness less than 475 µm), uncontrolled glaucoma or uveitis, significant cataract, functional monocularity, active eye inflammation or infections, severe dry eye, retinal holes, degenerative retina and macular disease.
Of course, no laser refractive procedures are recommended for pregnant or breastfeeding women because of possible refraction-altering hormonal changes.3 Also not acceptable are patients with a history of herpes simplex keratitis (unless risks of reactivation are controlled by antiviral treatment) and uncontrolled diabetes (because of delayed wound-healing concerns). To avoid surgical difficulties and poor outcomes, novice SMILE surgeons may postpone doing lower myopic corrections less than -2 D until they’ve gained some initial experience with at least 20 routine cases of SMILE with higher levels of preoperative myopia, difficult orbital anatomy and high astigmatism, as well as uncooperative and anxious patients, who can disrupt the still environment essential to successful SMILE procedures.1
When screening patients, use your slit lamp, pupillometer, corneal pachymetry, computed corneal topography and (if possible) computed videokeratography.4 Measure the patient’s manifest and cycloplegic refraction, refractive stability, and the degree of refractive error and astigmatism.
Besides applying diligent diagnostics to ensure candidates are appropriate for SMILE, you need to make sure they have realistic expectations. Review all risks and benefits, allowing plenty of time to answer questions and to educate them as you obtain informed consent. Patients should know what to expect to hear, feel, see and smell during the procedure, minimizing potential anxiety.
One point to cover with them is the possibility of some myopic regression or hyperopic surprise that may occur after SMILE because we’re trying to improve our nomograms over time. PRK can typically be used to correct this. LASIK with a thinner or thicker flap than the orginal SMILE cap is also an appropriate alternative. Some surgeons have the ability to convert the original SMILE cap into a LASIK flap.
Doing It Right
Immediately before SMILE, instill topical antibiotics and proparacaine 0.5%, in both eyes, but avoid using excessive amounts, which can loosen the epithelium and lead to surgically-induced black spots and epithelial defects.5
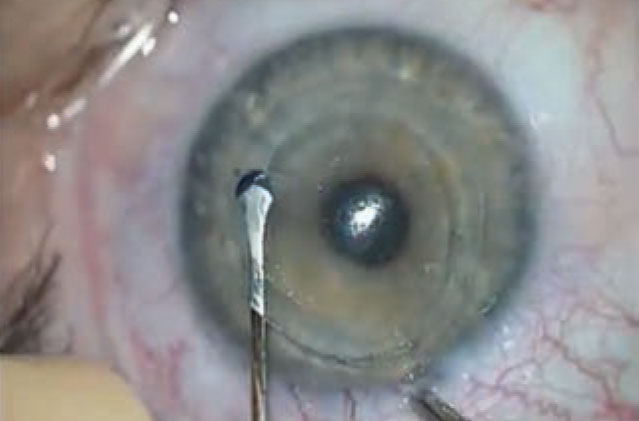 |
| Figure 2. Dissect with care. An untimely dissection into the posterior plane causes the lenticule to adhere anteriorly to the cap. |
Instruct patients to lie on their backs with their necks straight and legs uncrossed, remaining comfortable. Patient cooperation is critical. Provide specific instructions to help them minimize the risk of inadvertently disrupting two core functions of SMILE—docking and centration of the laser. They should:
• keep their head as still as possible;
• stay calm;
• fixate their eye on the blinking green light in the laser; and
• keep their eye wide open and still, resisting urges to close it.
After the patient is comfortably situated, you can dock the laser on the first eye and verify that it’s properly docked before contacting the cornea with the laser’s curved contact glass. Once the laser is centrated, initiate suction and maintain it for the duration of the procedure.
The femtosecond laser generates four sequential pulses that cut through the stromal tissue and create an intrastromal refractive lenticule measuring 6 mm to 6.5 mm in diameter. This is the small portion of cornea that creates the intended correction when it’s removed. You’ll need to use the laser to make a 3-to 5-mm incision along the superior or superotemporal regions of the cornea. After making all five incisions, which takes less than 24 seconds to complete, use a spatula to separate the residual lenticular appendages along the anterior and posterior planes of the intrastromal bed, and forceps to extract the lenticule.
Most Challenging Part
Extracting the lenticule is typically the most challenging part of the procedure when you’re starting out with SMILE. It’s better to identify the anterior plane of the lenticule and dissect the lenticule from the cap first, then proceed with the posterior dissection of the lenticule from the residual stromal bed. If you don’t recognize the correct tissue plane during removal, and inadvertently dissect the posterior plane, it can be challenging to find the anterior plane of the lenticule.
To avoid this complication, look carefully for the meniscus-shaped gap between the inner ring and the lenticule edge, commonly known as the meniscus sign. The meniscus sign will help you discern the posterior plane from the posterior lamellar channel. Meanwhile, another challenge can arise if you haven’t dissected the lenticule in the intrastromal tissue properly, which will compromise your ability to distinguish the lenticule edge from the anterior dissection plane. This can result in a loss of needed countertraction from the corneal stroma.1
As our experience with SMILE has progressed, we’ve seen the development of alternative approaches to lenticule extraction that can spare inexperienced surgeons the challenge of an inadequate result. For example, you can separate the lenticule edge from the overlying cap by employing a “push-up” technique, using an instrument with a Y-shaped tip to engage the lenticule edge and push it up from the stromal bed.
You can also use intraoperative anterior segment optical coherence tomography to identify the dissection planes, which will be hyperreflective, enabling you to more easily recognize where to dissect the tissue. In addition, you can turn to a newer method of lenticule extraction, called lenticuloschisis, in which you peel the lenticule away from surrounding stroma and extract it without using a tissue dissector. I haven’t personally used this technique. To use it, you would find the edge of the lenticule and then use the 25- or 27-gauge intravitreal forceps to grasp the edge of the lenticule from its bed in a clockwise or counter-clockwise direction, very similar to the maneuver that we commonly do during capsulorhexis in cataract surgery.
Because this alternative minimally manipulates the tissue, some surgeons say it can produce a smooth interface, earlier visual recovery and better visual quality in the immediate postop period.6
Occasionally, you may encounter a lenticule that you’re unable to remove. You can meet this challenge by converting the procedure to femtosecond lenticule extraction, or FLEx, a forerunner of SMILE. Using this alternative, you create a corneal flap and, instead of ablating the corneal stroma, you complete the intrastromal dissection and extract the refractive lenticule that way.7
Another alternative to standard lenticule extraction is to use customized surface ablation, although this has limitations, including postop haze.6 In very rare cases, when you can’t extract the lenticule, or when a lenticule remnant is retained in the stromal bed postoperatively, your patient may experience irregular astigmatism. For this complication, patients have undergone effective treatment with transepithelial phototherapeutic keratectomy.4
Some surgeons may inject the stromal pocket where the lenticule was just removed with special Vision Blue dye or dilute Kenalog and subsequent BSS irrigation to find out if they can see any irregularly stained stromal margin from where the original lenticule was dissected.
Once you’ve completed surgery, administer topical steroids (dexamethasone 0.1%) and topical fluoroquinolone eye drops (moxifloxacin 0.5%) several times daily. Topical corticosteroids may be used up to four weeks postoperatively. You also need to closely follow these patients in the weeks to months after SMILE.
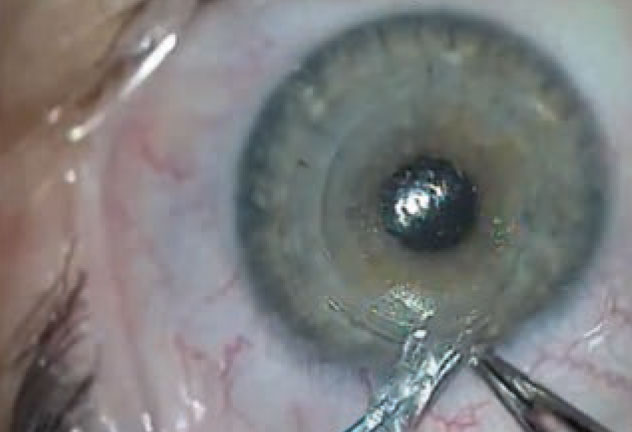 |
| Figure 3. Be careful to completely remove the lenticule, leaving no portion behind in the pocket. |
Managing Complications Safely
Besides the initial learning curve of SMILE, beginner surgeons must be familiar with potential intraoperative complications during the procedure.9 Below is what to watch for and how to respond proactively:
• Complications from lenticule creation. These can include suction loss, the formation of an opaque bubble layer, subconjunctival hemorrhage, incisional bleeding and black spots. Loss of suction occurs in about 6 percent of cases and is typically due to movement of the patient or the eye.1,9 If you lose suction when less than 10 percent of the lenticule has been cut, you can re-dock and re-centrate the laser. If more than 10 percent of the lenticule has been cut, you’ll need to convert to either PRK or LASIK. Most patients who experience suction loss and are appropriately treated for these complications experience excellent visual outcomes.5
The opaque bubble layer, secondary to the accumulation and opacification of bubbles in the intrastromal interface, can be managed intraoperatively by massaging them out of the interface. Use the same SMILE dissector or a spatula very similar in design to a cyclodialysis spatula. This complication can cause delayed visual recovery but doesn’t prevent good long-term visual outcomes.
The black spots are from debris or air bubbles that get trapped between the laser’s curved contact glass and the cornea. To eliminate them, clean the glass and, as needed, the ocular surface. This issue doesn’t typically affect visual outcomes except where the black spot was noted. Surgeons will have more difficulty with the dissection of the two planes form the actual lenticule and care must be taken to avoid lenticule or cap tear.5
• Complications from lenticule dissection or extraction. Watch for a lenticule remnant, corneal abrasion, lenticule adhesions and incisional tears.
A lenticule remnant and lenticule adhesions can be handled intraoperatively or postoperatively by using the techniques I discussed when addressing the most challenging part of SMILE. Peripheral corneal abrasions, caused by excessive manipulation in 5.5 percent of cases, are more common among surgeons who lack significant experience performing SMILE.8,9,10 Incisional tears occur in 9.6 percent of patients and may be secondary to surgeon inexperience or the patient suddenly moving the eye while an extraction instrument is inside of the SMILE pocket that was created by the laser. One way to avoid this is to get your patient to relax and to fixate his or her eyes intraoperatively.8 You can manage corneal abrasions and incisional tears with artificial tears and postop bandage contact lenses. They typically don’t affect visual outcomes.10
• Primary complications. Postop dry eye is found in about 3 percent of post-SMILE patients and can be attributed to decreased trophic influences in the corneal epithelium, inflammation, damage to limbal goblet cells during suction and impaired corneal sensation that enables blinking.7,10 Most studies report fewer dry-eye issues immediately after SMILE than after femtosecond LASIK, as demonstrated in higher levels of tear-film breakup time, corneal sensitivity and corneal nerve regeneration.10,6 Infectious keratitis, reported in some cases, can be avoided by using a postop antibiotic regimen. Affected patients, usually noncompliant with the postop treatment, should receive prompt irrigation with bactericidal povidone-iodine and an antibiotic solution.9
Other rare postop complications include epithelial ingrowth, irregular topography, microstriae and interface inflammation.7 Meanwhile, all known post-SMILE cases of ectasia have occurred in eyes with diagnosed or undiagnosed forme fruste keratoconus.11
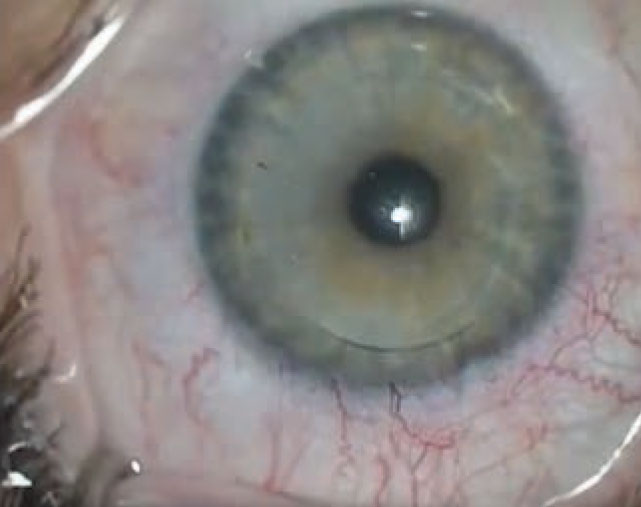 |
| Figure 4. Strict follow-up after SMILE is critical to avoid complications. Prescribe topical steroids and a fourth-generation fluoroquinolone, both q.i.d., for a week, followed by a three-week taper of steroids. The patient is seen after one day, one week, one month, three months and at one year. |
Is SMILE Worth Pursuing?
If you don’t provide SMILE to your patients, you may be asking yourself if it’s worth trying, considering the initial learning curve and potential risks that may intimidate surgeons who are new to the procedure. Indeed, there are challenging issues to consider. Patient discomfort during the initial learning phase of the SMILE may be higher but can be easily addressed with sufficient topical anesthesia, anxiolytics or sedatives.13 Postop light sensitivity and blurring of vision may affect some SMILE patients. Fortunately these issues typically resolve after three months.14
Visual recovery has been reported to be slower in SMILE, but I believe based on my experience that visual recovery for both procedures is very similar. (No significant difference between the two procedures has been documented at six months postoperatively.)6,7 However, when comparing LASIK to SMILE, we see several potential reasons to use SMILE, including long-term UDVA of 20/20 or better in 89 percent of post-SMILE patients,15 significantly fewer HOAs,1 good patient satisfaction,14,16 higher biomechanical stability of the postop cornea,14,16 fewer inflammatory cells in the cornea,9 less severe denervation and accelerated neuronal healing.7 Numerous meta-analyses have demonstrated that the long-term efficacy, predictability and safety outcomes of SMILE are comparable to those of femtosecond-LASIK.7,15
Because SMILE is a relatively new procedure, we need to await the results of additional clinical trials to validate these perceived advantages. Nonetheless, as I hope I’ve demonstrated, SMILE is a procedure that’s worth learning and one that won’t produce unacceptable complications if you proceed carefully, especially as you begin to climb that initial learning curve.
1. Moshirfar M, McCaughey MV, Reinstein DZ, et al. Small-incision lenticule extraction. J Cataract Refract Surg 2015;41:3:652-65.
2. Titiyal JS, Kaur M, Rathi A, et al. Learning curve of small incision lenticule extraction: Challenges and complications. Cornea 2017;36:11:1377-1382.
3. Shah R. History and Results; Indications and contraindications of SMILE compared With LASIK. Asia Pac J Ophthalmol (Phila) 2019;8:5:371-376.
4. Stein R. Photorefractive keratectomy. Int Ophthalmol Clin 2000 Summer;40:3:35-56.
5. Titiyal JS, Kaur M, Shaikh F, et al. Small incision lenticule extraction (SMILE) techniques: Patient selection and perspectives. Clin Ophthalmol 2018;12:1685-1699.
6. Ganesh S, Brar S, Arra RR. Refractive lenticule extraction small incision lenticule extraction: A new refractive surgery paradigm. Indian J Ophthalmol 2018;66:1:10-19.
7. Hamed AM, Heikal MA, Soliman TT, Daifalla A, Said-Ahmed KE. SMILE intraoperative complications: Incidence and management. Int J Ophthalmol 2019;12:2:280-283.
10. Krueger RR, Meister CS. A review of small incision lenticule extraction complications. Curr Opin Ophthalmol 2018 Jul;29:4:292-298.
11. Wang Y, Ma J, Zhang J, et al. Incidence and management of intraoperative complications during small-incision lenticule extraction in 3004 cases. J Cataract Refract Surg 2017;43:6:796-802.
12. Wong AHY, Cheung RKY, Kua WN, et al. Dry eyes after SMILE. Asia Pac J Ophthalmol (Phila). 2019 Sep-Oct;8:5:397-405.
13. Moshirfar M, Albarracin JC, Desautels JD, et al. Ectasia following small-incision lenticule extraction (SMILE): A review of the literature. Clin Ophthalmol. 2017;11:1683-1688.
14. Chiam NPY, Mehta JS. Comparing patient-reported outcomes of laser in situ keratomileusis and small-incision lenticule extraction: A review. Asia Pac J Ophthalmol (Phila) 2019;8:5:377-384.
15. Li M, Li M, Chen Y, et al. Five-year results of small incision lenticule extraction (SMILE) and femtosecond laser LASIK (FS-LASIK) for myopia. Acta Ophthalmol 2019;97:3:e373-e380.
16. Dishler JG, Slade S, Seifert S, et al. Small-Incision Lenticule Extraction (SMILE) for the correction of myopia with astigmatism: Outcomes of the United States Food and Drug Administration premarket approval clinical trial. Ophthalmology 2020;127:8:1020-1034.
17. Klokova OA, Sakhnov SN, Geydenrikh MS, et al. Quality of life after refractive surgery: ReLEx SMILE vs Femto-LASIK. Clin Ophthalmol 2019;13:561-570.



