The list of new options for managing glaucoma keeps growing, and stents designed to aid outflow are high on the list. Here, surgeons share their thoughts and experience with three new implant devices under investigation: the CyPass Micro-Stent; the Hydrus Microstent; and the AqueSys.
The CyPass Micro-Stent
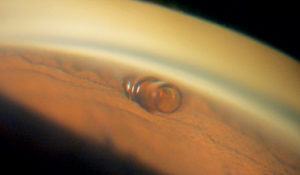
The CyPass (Transcend Medical, Menlo Park, Calif.) is a supraciliary device designed to create a controlled outflow pathway to the suprachoroidal space. The device is a 6.35-mm long tube with an outer diameter of 0.51 mm; it’s made of a polyimide material similar to that used in intraocular lens haptics, and it can be placed through a 1.5-mm corneal incision. It’s inserted on a small guidewire with a special tip that separates the iris from the scleral spur; the device is inserted into the cleft that’s created. Once in place, openings all along the length of the tube allow aqueous to flow out. The device already has CE Mark approval.
|
A 2010 European study of the CyPass involving 81 subjects found that mean intraocular pressure decreased from 22.9 to 16.2 mmHg at six months, with the number of medications used dropping from 1.9 to 1.3, following implantation. Subjects with a baseline IOP greater than 21 mmHg saw an average 35 percent drop in pressure (30.5 to 19.9 mmHg). Intraoperatively there were two cases of hyphema that resolved by one week, and one case of a shallowed anterior chamber that resolved by one month.
Steven D. Vold, MD, founder of VoldVision in Springdale, Ark., has been implanting the CyPass since 2009 as part of its prospective Food and Drug Administration clinical trial (COMPASS). “In the COMPASS trial, patients with mild to moderate POAG and visually significant cataracts are randomized to treatment with cataract surgery alone vs. cataract surgery combined with the CyPass,” he explains. “Early data demonstrates IOP lowering of approximately 35 to 40 percent and a significant reduction in the need for topical glaucoma medication at six months.
“Thus far,” he continues, “it appears to have an outstanding patient safety profile. My early personal experience parallels these findings. Postoperative recovery following CyPass microstent placement combined with cataract surgery is very similar to that observed with cataract surgery alone.”
Kuldev Singh, MD, MPH, professor of ophthalmology and director of the Glaucoma Service at Stanford University School of Medicine believes the CyPass is the most promising among the minimally invasive procedures that drain aqueous humor into the suprachoroidal space. “Having access to this pathway with an ab interno approach is exciting,” he says. “I eagerly await the results of the pivotal study.”
The Hydrus Microstent
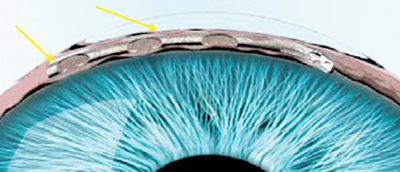
The manufacturer of the Hydrus, Ivantis (Irvine, Calif.), describes it as an “intracanalicular scaffold.” The device, about the size of an eyelash, is made from nitinol, a highly elastic, biocompatible alloy used in many implantable medical devices. The Hydrus is placed inside Schlemm’s
canal during cataract surgery using the cataract incisions; it increases outflow by allowing aqueous to bypass the trabecular meshwork, and by dilating the canal. The company says the device’s non-luminal open design improves flow into the canal and gives it better access to collector channels.
|
“The Hydrus dilates Schlemm’s canal for approximately three clock hours in the nasal quadrant, thereby enhancing aqueous outflow,” notes Dr. Vold. “During the past three months, I’ve placed these devices in Mexico and the United States as part of its pivotal prospective FDA clinical trial, which is evaluating mild to moderate POAG patients with visually significant cataracts. Early six-month data demonstrates IOP lowering from the low 20s to the mid-teens with almost complete elimination of topical medication use. Thus far, no significant device issues have been encountered.”
Dr. Singh believes that this approach is a logical way to increase outflow via conventional pathways. “Initial communication from European colleagues is that this device works very well,” he says. He adds that with appropriate training, the implantation of either the Hydrus or CyPass devices shouldn’t be difficult for the anterior segment surgeon.
Ivantis is currently enrolling patients for a large international study, the Hydrus IV, which will compare patients undergoing cataract surgery with and without the Hydrus shunt.
The AqueSys
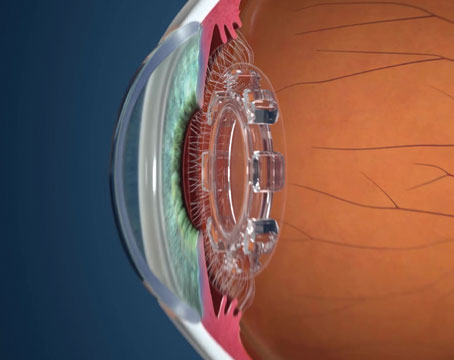
The AqueSys system is described as the world’s first ab interno subconjunctival approach for lowering IOP. It involves a soft, flexible, permanent gelatin implant, about the diameter of a human hair. The implant procedure can be done alone, or as part of cataract surgery. The implant is placed through a small, self-sealing corneal incision using an inserter; it’s implanted into the subconjunctival space opposite the incision. The gelatin material is non-inflammatory, exerts minimal stress on surrounding tissue and doesn’t migrate once placed. The surgery doesn’t disrupt the conjunctiva, so it leaves all other options open for future use if needed, and it can be repeated.
|
“The AqueSys is made of collagen that’s likely to cause minimal corneal endothelial trauma compared to other silicone or steel implants,” says Rohit Varma, MD, MPH, director of the glaucoma service, ocular epidemiology center and clinical trials unit at the USC Keck School of Medicine in Calif. “Of all the implants that I’ve seen and used to date, the AqueSys Implant is the easiest to implant, because it uses an ab interno approach that does not incise the conjunctiva or subconjunctival tissues. On average, it leads to a reduction of 6 to 8 mmHg in IOP at 12 months of follow-up.”
Dr. Vold notes that the FDA just approved AqueSys’s 510(k) protocol for the treatment of refractory glaucoma. “I’ll be implanting these devices in the United States during the next few weeks,” he says. “Early experience showed the development of low, diffuse, posterior blebs.” REVIEW
Dr. Singh has served as a consultant for Ivantis and Transcend Medical. Dr. Vold is a consultant for Transcend Medical, and Dr. Varma is a consultant to AqueSys.