Presentation and Initial Work-up
An 18-year-old white male presented with 10 years of high refractive error requiring high correction spectacles. The patient reported that he had previously tried contact lenses and couldn’t tolerate them. He reported that he was going to college in the coming months and felt socially isolated because of the strength of his spectacles.
Ocular examination demonstrated best corrected visual acuity with correction of 20/20-2, J3 in his right eye and 20/30, J1 in his left eye. His current spectacle prescription was +9.50 + 1.50 x 088 in the right eye and +10.75 + 0.50 x 085 in the left eye. Pupils were 6 mm in the right and 6.5 mm in the left and equally reactive to light and accommodation. There was no afferent pupillary defect. Intraocular pressures were 10 and 15 mmHg in the right and left eyes, respectively. Confrontation visual fields were full in both eyes. Extraocular motility was full bilaterally.
Adnexal, eyelid, conjunctival and corneal exams were unremarkable. The anterior chamber exam in the right eye was notable for vitreous strands to the cornea, but was otherwise quiet. The left eye’s anterior chamber was quiet. The lens exam demonstrated aphakia with a capsular opening of 6 mm and a Sommerings ring cataract in the right eye; and aphakia with a capsular opening of 4.5 mm, Elshnig pearls and capsular remnant in the left eye.
Medical History
Past ocular history included idiopathic panuveitis associated with eosinophilia over 10 years ago which necessitated pars plana vitrectomy followed by cataract extraction in both eyes. The patient also had congenital glaucoma in both eyes, more severe in the left eye than the right, requiring a goniotomy in the left eye eight years prior. The idiopathic eosinophilia had been quiet, and the patient had been off of systemic medications for more than five years. Social and family history were both unremarkable. The patient’s currently prescribed ocular medications included latanoprost before bedtime and brimonidine twice a day, both in the left eye, which he last used more than three years ago, as the patient had been lost to follow-up.
What is your diagnosis? What further work-up would you pursue? The diagnosis appears below.
Work-up, Diagnosis and Treatment
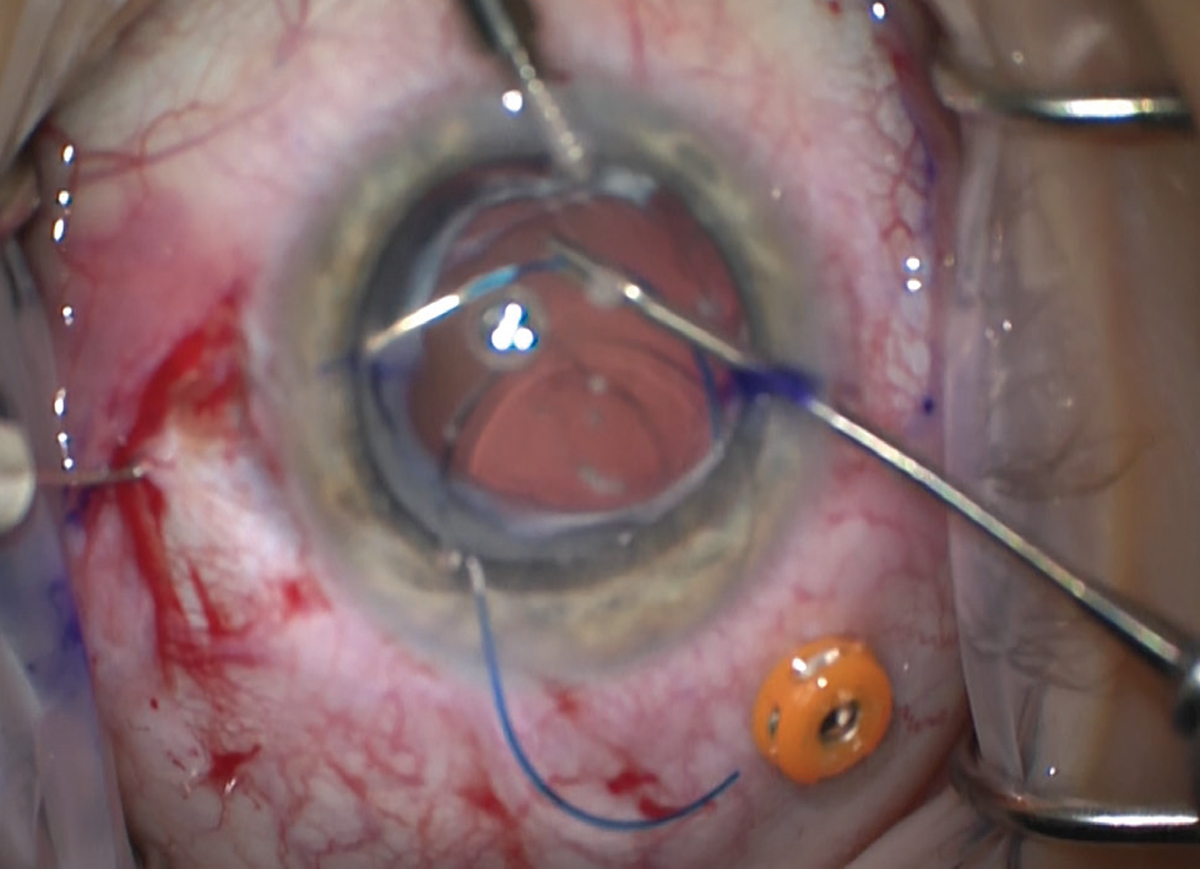 |
|
Figure 1. The haptics are docked into thin-walled, 30 gauge needles with microinstruments before being externalized. |
Additional in-office testing was obtained including optical coherence tomography of the macula and optic nerve which were both normal other than mild thinning of the left optic nerve. Humphrey visual field 30-2 stimulus III was normal in both eyes. Surgical preoperative testing demonstrated mild with-the-rule astigmatism in both eyes and mild internal optical aberrations in both eyes. Ancillary laboratory testing, including complete blood count with differential, erythrocyte sedimentation rate, C-reactive protein, angiotensin converting enzyme, perinuclear anti-neutrophil cytoplasmic antibodies (P-ANCA), cytoplasmic anti-neutrophil cytoplasmic antibodies (C-ANCA), rheumatoid factor and QuantiFeron gold tests, were obtained. These tests were all normal.
The patient was observed for one year, during which his intraocular pressures were normal off drops and his anterior and posterior chambers remained quiet. Surgical options for this patient included sulcus fixation with posterior optic capture, anterior chamber intraocular lenses, iris-sutured posterior chamber IOLs and scleral-fixated IOLs. The left eye underwent surgery with an attempt at placement of a sulcus IOL with posterior optic capture, but this was unsuccessful due to the nature of the residual capsule, so the decision was made to proceed with intrascleral haptic fixation using the Yamane technique.1 The haptics were externalized with thin-walled, 30-gauge TSK needles (Figure 1) and cauterized to create anchoring bulbs (Figure 2). The left eye remained quiet with normal intraocular pressures for two months at which point the right eye underwent the same procedure. Of note, this patient had thick Tenon’s and conjunctiva, so the surgeon created a peritomy for ease of haptic placement. Final best-corrected visual acuity in both eyes was 20/20, intraocular pressures were normal, and anterior chambers and vitreous were quiet two months following the right eye surgery. Final spectacle correction was +0.25 + 1.50 x 087 + 2.50 in the right eye and -0.150 + 1.50 x 093 + 2.50 in the left eye. The patient and his parents reported dramatic improvement in his social interactions and quality of life.
Discussion
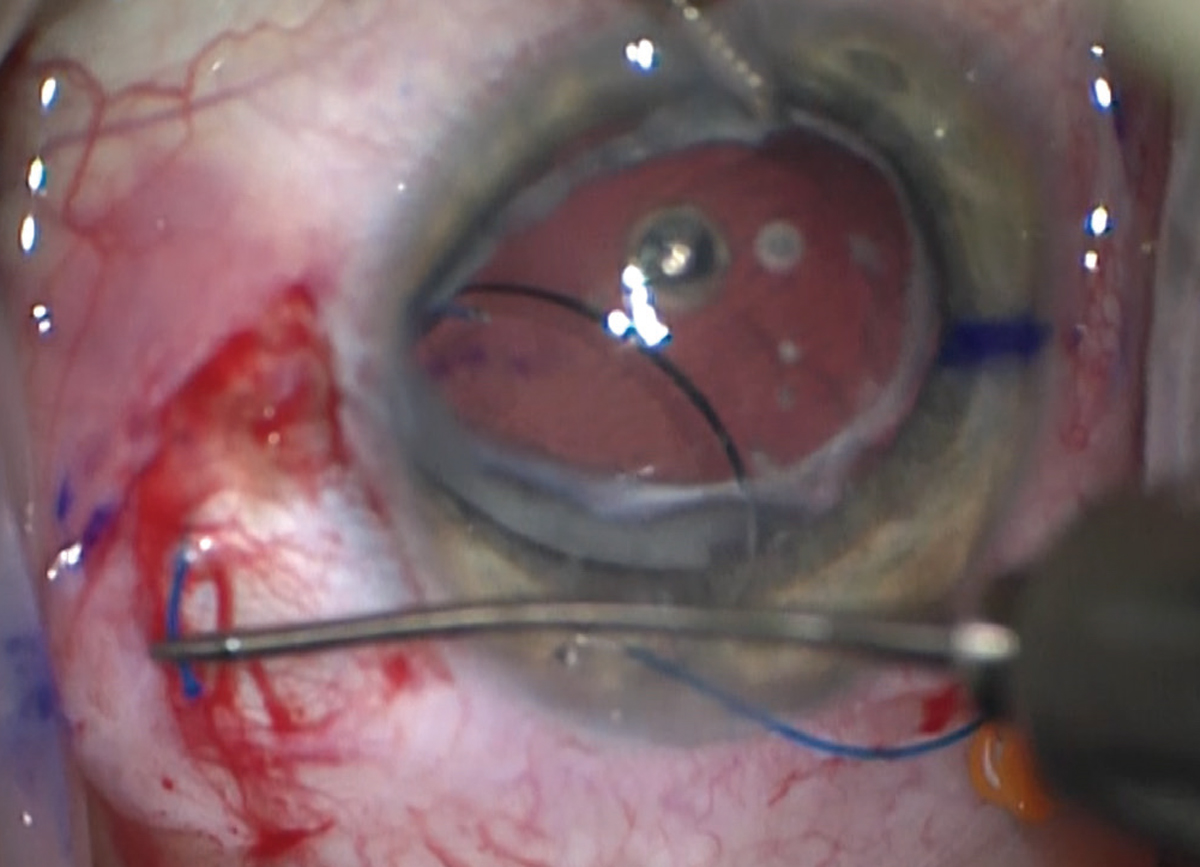 |
| Figure 2. Low-tension cautery is used to create a terminal bulb on the end of the haptic, such that it will stay external to the eye. |
In patients with adequate capsular support the preferred approach is almost always to maintain the capsule and secondarily implant an intraocular lens. For a variety of reasons this support may not exist, at which point the surgeon must consider other appropriate means of IOL fixation. As mentioned, the options available to the anterior segment surgeon include AC IOLs, iris-sutured posterior chamber IOLs and scleral-fixated IOLs. Each of these has different benefits and risks which should be carefully considered prior to surgical intervention.
AC IOL implantation, in comparison to other aphakic lens options, is a relatively simple procedure for the surgeon. That said, the question of their long-term safety is a concern. Corneal endothelial decompensation is an especially concerning consequence that may occur in up to half of eyes over the course of the 12 years following surgery, according to one study.2 They also require a larger surgical incision and close patient follow-up, given the proximity to angle structures and resulting concern for secondary inflammation and glaucoma.3,4 Uncontrolled vitreous loss during surgery and subsequent sequelae is another significant concern.
Further posteriorly, iris-sutured IOLs permit a smaller incision. They share the inflammatory risk seen with AC IOLs, but given their more posterior location they don’t carry the same risk of endothelial decompensation seen with AC IOLs. However, their anatomical location does bring increased risk for iris chafing, with resultant pigment dispersion and secondary glaucoma, as well as irregular pupils or pupillary dysfunction.3,5
In order to avoid corneal endothelium, angle and iris structures, the surgeon can employ scleral-fixation techniques. These are technically more challenging procedures but, especially in pediatric patients like ours, the benefits may outweigh the risks. IOLs may be fixated to the sclera either with or without sutures.3 Sutured fixation techniques have been considered an effective method to approach the aphakic pediatric eye, although long-term adverse effects related to the sutures have been reported, including suture erosion and breakage with resultant IOL dislocation.6 This adverse event has been seen most commonly with 10-0 polypropylene suture fixated IOLs.4 The mechanism of breakage has been found to be secondary to the sharp edges of IOL positioning holes cutting the sutures and subsequent IOL subluxation.7 Breakage of polypropylene sutures led to the search for a more reliable material such as Gore-Tex. Gore-Tex sutures may have a favorable safety profile for multiple reasons (they’re braided and have a higher tensile strength than comparable monofilaments). Gore-tex is off-label, but has been used successfully for over a decade now. However, longer-term studies would need to be done to follow the performance of such alternative suture types over multiple decades.8
Various methods of sutureless scleral fixation techniques have recently become increasingly popular, given concerns about long-term suture reliability, especially in pediatric patients in whom IOL longevity is critical. Fixation by means of fibrin glue is one such method. One study followed forty-one eyes of 33 pediatric patients in which IOL haptics were externalized through partial-thickness scleral flaps which were then closed with fibrin glue. Within the mean follow-up of 17.5 months, two IOLs underwent decentration with an otherwise favorable side-effect profile.9
More recently, a flanged technique developed by Dr. Yamane and his co-workers, which was used in our case, has gained popularity.1,10 This technique uses thin-walled, 30-gauge needles to externalize IOL haptics through the sclera, with subsequent cauterization of the externalized haptics. This creates a terminal bulb on the haptics, preventing them from regressing back into the globe. This technique is commonly referred to as intrascleral haptic fixation (ISHF), or the double-needle technique. The original study of the technique in 2017 detailed 100 eyes of 97 consecutive patients over 36 months and included complications of iris capture in eight eyes (8 percent), vitreous hemorrhage in five eyes (5 percent), and cystoid macular edema in one eye (1 percent).1
Since its original publication, multiple variations on this flanged technique have been proposed, including modifications to needle docking, trocar-assisted docking, flattened flanges, refixation and double-flanged techniques.10 These variations have varying technical difficulties and require further study but are based on the principle of externalizing IOL haptics with subsequent cauterization. One benefit of this technique is that the experienced surgeon may readily modify the IOL position or explant the IOL by simply amputating the terminal bulb, internalizing the haptic or suture and adjusting as needed. This is of particular importance in a patient with a long lifetime ahead.
The pediatric aphakic patient has a number of options which continue to evolve as surgeons seek to optimize patient outcomes. Anterior chamber and iris-sutured IOLs may be technically easier but come with the risk of contact with anterior chamber structures such as corneal endothelium, angle and the iris. Scleral-fixated techniques seek to avoid these structures but may present additional technical challenges. They also require careful preoperative planning to select the technique which the surgeon can comfortably perform, but presents the best potential outcome for the patient.
1. Yamane S, Sato S, Maruyama-Inoue M, Kadonosono K. Flanged intrascleral intraocular lens fixation with double-needle technique. Ophthalmology 2017;124:8:1136-1142.
2. Tang Y, Xu J, Chen J, Lu Y. Long-term destiny of corneal endothelial cells in anterior chamber intraocular lens-implanted eyes. J Ophthalmol 2020;Dec 24 (online article). doi:10.1155/2020/5967509
3. Terveen DC, Fram NR, Ayres B, Berdahl JP. Small-incision 4-point scleral suture fixation of a foldable hydrophilic acrylic intraocular lens in the absence of capsule support Presented at the ASCRS Symposium on Cataract, IOL and Refractive Surgery, San Diego, California, USA, April 2015. J Cataract Refract Surg 2016;42:2:211-216.
4. Karasavvidou EM, Wilde C, Zaman A, Orr G, Kumudhan D, Panos GD. Surgical management of paediatric aphakia in the absence of sufficient capsular support. J Ophthalmol. 2021;Dec 4 (online article). doi:10.1155/2021/2253486
5. Condon GP, Masket S, Kranemann C, Crandall AS, Ahmed IIK. Small-incision iris fixation of foldable intraocular lenses in the absence of capsule support. Ophthalmology 2007;114;7:1311-1318.
6. Cheung CSY, VanderVeen DK. Intraocular lens techniques in pediatric eyes with insufficient capsular support: Complications and outcomes. Semin Ophthalmol 2019;34:4:293-302.
7. Parekh P, Green WR, Stark WJ, Akpek EK. Subluxation of suture-fixated posterior chamber intraocular lenses. A clinicopathologic study. Ophthalmology 2007;114:2:232-237.
8. Khan MA, Gupta OP, Smith RG, et al. Scleral fixation of intraocular lenses using Gore-Tex suture: Clinical outcomes and safety profile. Br J Ophthalmol 2016;100:5:638-643.
9. Kumar DA, Agarwal A, Prakash D, Prakash G, Jacob S, Agarwal A. Glued intrascleral fixation of posterior chamber intraocular lens in children. Am J Ophthalmol 2012;153:4:594-601.
10. Yamane S, Ito A. Flanged fixation: Yamane technique and its application. Curr Opin Ophthalmol 2021;32:1:19-24.



